Introduction
Marine organism-derived bioactive peptides have been obtained by enzymatic hydrolysis of marine proteins. These peptides have many biological functions such as antihypertensive (1), anti-cancer (2), anti-microbial (3), antioxidant (4), and anti-inflammatory (5) activities. Bioactive peptides are usually composed of 3-20 amino acid residues, and their various activities are dependent on amino acid composition and sequence (6).
Oxidative stress caused by lipid oxidation contributes to the development and progression of diseases such as diabetes mellitus, neurodegenerative, cancer, and inflammatory diseases (7). In addition, lipid oxidation in foods causes flavor and texture alterations as well as nutrition loss, leading to shortened shelf-life of food products (8). Accordingly, many synthetic antioxidants such as propyl gallate (PG), butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT) are widely used in the food industry to preserve food quality. However, use of large amounts of synthetic antioxidants causes stability problems in foods. Therefore, the identification of safe natural antioxidants to replace synthetic compounds is an important issue.
Many studies have already attempted the isolation and extraction of antioxidants (phenolic acid, flavonoids, carotenoids, and peptides) from natural materials such as grains, medicinal plants, vegetables, fruits, and fishes. Among others, antioxidant peptides isolated from products with abundant protein contents have been reported frequently in recent studies. For example, peptides isolated from protein hydrolysate of Pseudosciaena crocea was shown to exert antioxidant effects (9). Chi et al. (10,11) reported three antioxidant peptides isolated from protein hydrolysate of bluefin leatherjacket skin and head, and Kangsanant et al. (12) investigated antioxidant peptides purified from Tilapia protein hydrolysate. Additionally, many researchers have identified antioxidant activity in peptides isolated from anchovy (13), sandfish (14), Mideodeok (15), chickpea (16), and bovine hair (17) protein hydrolysates. These results suggest that antioxidant peptides isolated from protein hydrolysates may be effectively used as food additives and pharmaceutical excipients (8).
Sandfish, Arctoscopus (A.) japonicus, is a popular food in South Korea. It migrates to the East Sea in late autumn, spawning in shallow waters (2-10 m in depth) in the winter (18). During this spawning season from November to December, many A. japonicus are captured and consumed (19). Catches of A. japonicus are on an increasing trend, with catches from 4,236 M/T in 2010 to 4,762 M/T in 2015 (20). In particular, A. japonicus is caught in large quantities in the East Sea of Korea, and, it is superabundant due to falling consumption. Therefore, it is necessary to utilize superabundant A. japonicus as a material for food processing. In addition, roe of A. japonicus is rich in protein, and its protein hydrolysate was able to inhibit lipid oxidation in a preliminary study. Nevertheless, there is insufficient information about bioactive peptide derived from A. japonicus roe protein hydrolysate. Thus, the present study was undertaken to isolate antioxidative peptide from A. japonicus roe and present the feasibility as materials for manufacturing functional foods and pharmaceutical products.
Materials and Methods
Sandfish (A. japonicus, 90±10 g in weight and 18.0±2.2 cm in length) was purchased from a wholesale market located in Daegu, South Korea and transported on ice to the laboratory. The roe was separated from sandfish and grinded in a food mixer (M-1211, Starion, Busan, Korea). The temperature was maintained a constant during grinding in order to minimize the quality changes in roe, and the grinded samples were stored in a deep freezer (MDF-435, Sanyo, Tokyo, Japan) at -40℃.
Before protein hydrolysis, roe of sandfish was defatted as followed by Klompong et al. (21) with some modifications. The grinded roe was stirred with isopropyl alcohol (Sigma Chemical, St. Louis, MO, USA) (1:4, w:v) for 50 min at room temperature. After centrifugation at 700 ×g for 30 min, the supernatant was discarded, and the precipitate was collected and lyophilized using a freeze dryer (FD-1, Eyela, Tokyo, Japan) for 5 days.
Results of our preliminary antioxidant experiments using different commercial proteases (Alcalase 2.4 L, Collupulin MG, Flavourzyme 500MG, Neutrase 0.8 L, and Protamex) (Novo Nordisk Co., Bagsvaerd, Denmark) showed that the antioxidant activity of the hydrolysate obtained using Collupulin MG was the highest among all hydrolysates. In addition, the results of our preliminary study indicated that the hydrolysate obtained using Collupulin MG recorded its highest antioxidant activity at pH 9.0 with a hydrolysis temperature of 60℃, enzyme/substrate ratio of 5% (w/w), and hydrolysis time of 1 h. Thus, the following hydrolysis conditions were used in the present study: defatted roe was mixed with distilled water at a ratio of 1:10 (w:v) and boiled at 90℃ for 20 min to inactivate endogenous enzymes. The pH of the mixture was adjusted to 9.0, and enzyme was added to the reaction mixture at a final activity of 1,150 U (enzyme/substrate ratio of 5%). The solution was incubated at 60℃ for 1 h and then boiled at 90℃ for 20 min to inactivate the enzyme. Next, the mixture was centrifuged at 1,600 ×g for 30 min to isolate the hydrolysate. The hydrolyzed supernatant was collected, lyophilized for 5 days, and stored at -40℃ for future analysis.
The Measurement of DH was followed by Sathivel et al. (22) with a slight modification. The SRH was mixed with an equal amount of 20% trichloroacetic acid (TCA) (Sigma Chemical) to obtain 10% TCA-soluble protein, followed by centrifugation at 1,600 ×g for 30 min. TCA-soluble protein in the supernatant was analyzed using the bicinchoninic acid (Sigma Chemical) method (23) with bovine serum albumin (Sigma Chemical) as the standard. The DH was calculated as follows:
In the preliminary study, the highest DPPH radical scavenging activity was observed in crude hydrolysate, relatively to ABTS radical scavenging activity, hydroxyl radical scavenging activity, nitrite scavenging activity, and metal chelating activity. Therefore, DPPH radical scavenging activity was selected to estimate antioxidant activity of the fractions. DPPH radical scavenging activity was measured according to the method described by Jang et al. (14) with slight modifications. Sample (1 mL) was added to 0.5 mL of 0.2 mM 1,1-diphenyl-2-picryl hydrazyl (DPPH, Sigma Chemical). The mixture was shaken for 5 sec and incubated in a water bath at 37℃ for 30 min. Absorbance was then measured at 517 nm with a spectrophotometer (U-2900, Hitachi Ltd., Tokyo, Japan). Radical scavenging activity was calculated as follows:
where Acontrol is the absorbance of the control (blank) and Asample is the absorbance of the hydrolysate.
To isolate antioxidant peptides from the SRH, the SRH was fractionated using an UF system with molecular weight cut-off (MWCO) values of 3, 5, and 10 kDa. The SRH fraction was divided into four parts designated as > 10 kDa, 5-10 kDa, 3-5 kDa, and < 3 kDa. All fractions were lyophilized and their molecular weight distribution and antioxidant activity evaluated. The degree of molecular weight distribution was expressed as the relative percentage of each yield.
The fraction with the highest antioxidant activity was separated on a XBridge PST C18 preparative column (10 mm× 250 mm, Waters Co., Milford, MA, USA) connected to an HPLC system (Waters 2695, Waters Co.). The solvents used were 0.1% (v/v) trifluoroacetic acid (TFA) (Sigma Chemical) in distilled water (solvent A) and 0.1% (v/v) TFA in 80% acetonitrile (solvent B). The flow rate was 5.0 mL/min with a linear gradient of 0-50% solvent B in 30 min. The loaded fraction was divided into six fractions by an automatic Foxy Jr. fraction collector (Teledyne Isco, Lincoln, NE, USA). The activity of each fraction was measured using DPPH radical scavenging activity assay. The fraction exhibiting the strongest antioxidant activity was pooled and lyophilized.
The fraction collected by prep-HPLC was loaded onto an AtlantisTM dC18 column (4.6 mm×150 mm, Waters Co.) connected to a HPLC system (Waters Co.). The solvents used (solvent A and solvent B) were prepared as described above. The flow rate was 0.8 mL/min with a linear gradient of 0-100% solvent B in 15 min. DPPH radical scavenging activities of the loaded fractions were measured. The fraction exhibiting the highest antioxidant activity was pooled and lyophilized. The fraction was fractionated once again by RP-HPLC so only one peak was observed. The analytical conditions were the same as those already described. The isolated antioxidant peptide (IAP) used for all follow-up experiments was pooled and lyophilized.
Results of the study are presented as the mean and standard deviation (mean±SD). All experiments were performed in triplicate. Collected data were subjected to analysis of variance (ANOVA), and Duncan’s multiple range test was used to identify significant differences (p<0.05). All statistical analyses were carried out using SPSS ver. 21.0 software (SPSS Inc., Chicago, IL, USA).
Results and Discussion
The DH of the hydrolysate affects processing of food products since functional properties such as protein solubility, emulsification, and foaming are susceptible to the DH. For instance, hydrolysate exhibiting high solubility can be easily mixed with other ingredients and displays superior wetting when stirred into liquids (14). Moreover, the DH influences the biological properties of peptides produced during hydrolysis due to its effects on amino acid composition as well as peptide size or structure (21,24). In this study, the DH of the SRH under optimum hydrolysis conditions was 55.07% (data not shown). Similar observations were reported in another study. Ovissipour et al. (25) reported DH values of more than 53% for the protein hydrolysate obtained from yellowfin tuna visceral waste. In addition, Bhaskar et al. (26) and Bhaskar and Mahendrakar (27) reported that the DH of the protein hydrolysate obtained from Catla viscdral waste was close to 50%. Souissi et al. (24) reported that hydrolysates with low DH values contain highly hydrophobic peptides, whereas hydrolysates with high DH values include highly hydrophilic peptides. Therefore, the results of the present study are expected to impact the various characteristics of peptides, which will be isolated later on.
Molecular weight is one of the most important factors affecting production of protein hydrolysates with expected functional properties for use as biomaterials (28). Accordingly, the degree of molecular weight distribution for the four fractions (> 10 kDa, 5-10 kDa, 3-5 kDa, and < 3 kDa) was obtained by UF (Table 1), and the antioxidant activities of the different molecular weight fractions were determined (Fig. 1).
| Molecular weight | Distribution (%) |
|---|---|
|
|
|
| SRH1) | |
| >10 kDa | 25.75±1.852)b3) |
| 5-10 kDa | 17.15±0.87c |
| 3-5 kDa | 19.59±2.30c |
| <3 kDa | 37.51±1.29a |
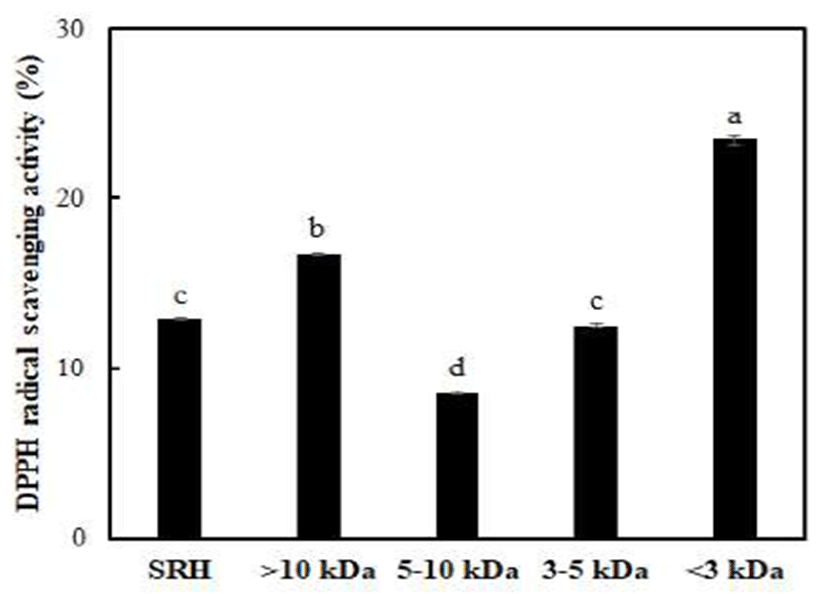
The SRH with a molecular weight below 3 kDa constituted about 38% of the whole hydrolysate. This indicates that the SRH contained mostly low molecular weight peptides. Similar results were reported by You et al. (29) in a study on antioxidant peptide derived from loach (Misgurnus anguillicaudatus). The fraction with a molecular weight below 3 kDa showed significantly greater antioxidant activity compared to the original SRH and other fractions. The antioxidant activity of SRH tended to increase with decreasing molecular weight, whereas the antioxidant activity of the fraction with a molecular weight above 10 kDa was the second highest after the fraction with a molecular weight below 3 kDa. You et al. (30) reported a peptide with a molecular weight less than 3 kDa showing the strongest hydroxyl radical scavenging activity. Chi et al. (9-11) also identified a fraction with a molecular weight below 1 kDa, which is the smallest molecular weight, with the highest antioxidant activity. However, Kim et al. (31) demonstrated that free radical scavenging activities of the smallest molecular weight fraction were lower than those of the fraction with a molecular weight of 5-10 kDa. In addition, Zhou et al. (32) reported that a fraction with a molecular weight of 3-10 kDa more effectively scavenged DPPH radicals than a fraction with a molecular weight below 1 kDa. These results suggest that the antioxidant activity according to molecular weight differs by sample type. In the present study, the fraction with a molecular weight below 3 kDa, which showed the highest radical scavenging activity, was pooled and lyophilized for subsequent studies.
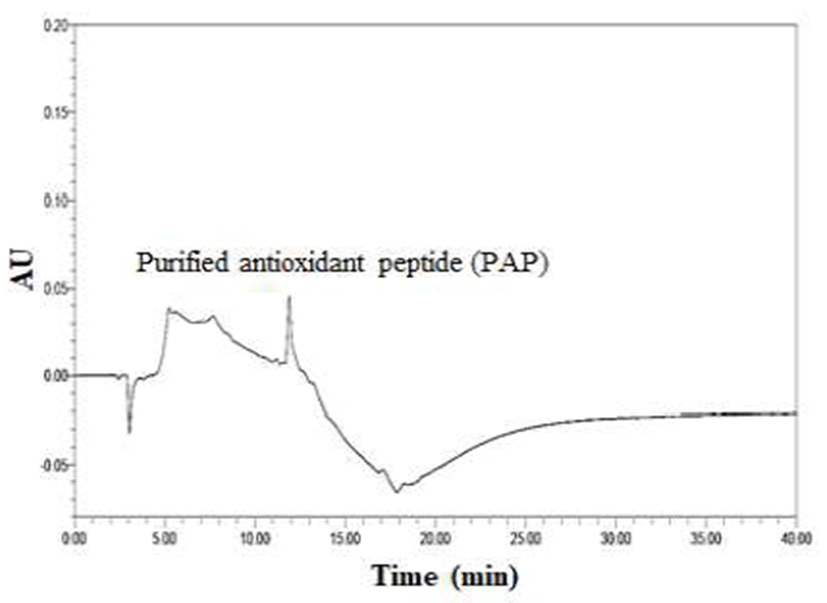
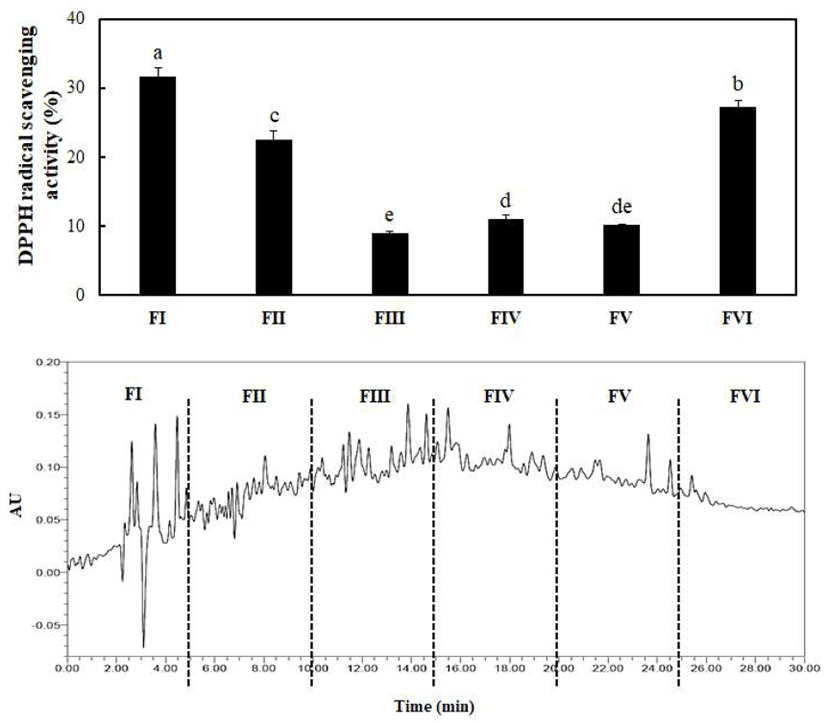
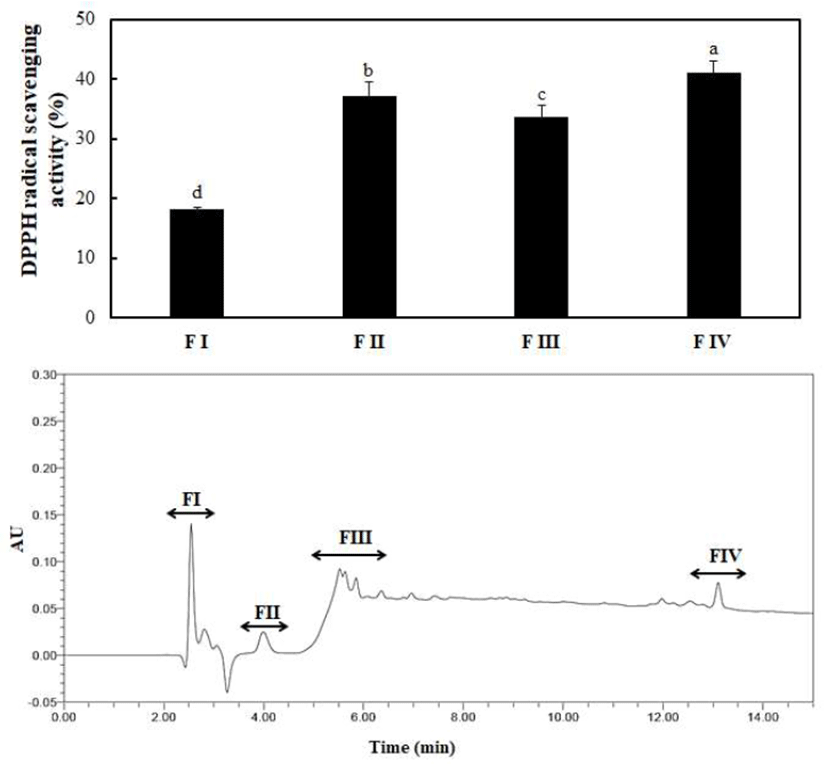
In Fig. 2, the column chromatographic profiles and DPPH radical scavenging activities are presented. A clear difference was observed between the fractions, and the scavenging activities of fractions I, II, III, IV, V, and VI were 31.72%, 22.54%, 8.96%, 10.97%, 10.08%, and 27.25%, respectively. Briefly, fraction I exerted the strongest antioxidant activity among all fractions. Therefore, fraction I obtained by prep-HPLC was loaded onto a dC18 column for RP-HPLC and further divided into four major portions. The order of radical scavenging effects on the four fractions was FI < FIII < FII < FIV (Fig. 3). The DPPH radical scavenging activity of fraction IV was the highest (41.10%) among all fractions. The fraction was continuously loaded again onto the same reverse-phase column and estimated under the same analytical conditions. Eventually, only a single peak was obtained, and a fraction with a DPPH radical scavenging activity of 91.25% was eluted (Fig. 4 and Table 2). The purification fold of the IAP from SRH throughout the four-step procedure was 7.11-fold, and protein yield was 14.8%.
Isolation fold was calculated as a ratio of the radical scavenging activity of fraction obtained by each step relative to the radical scavenging activity of SRH.
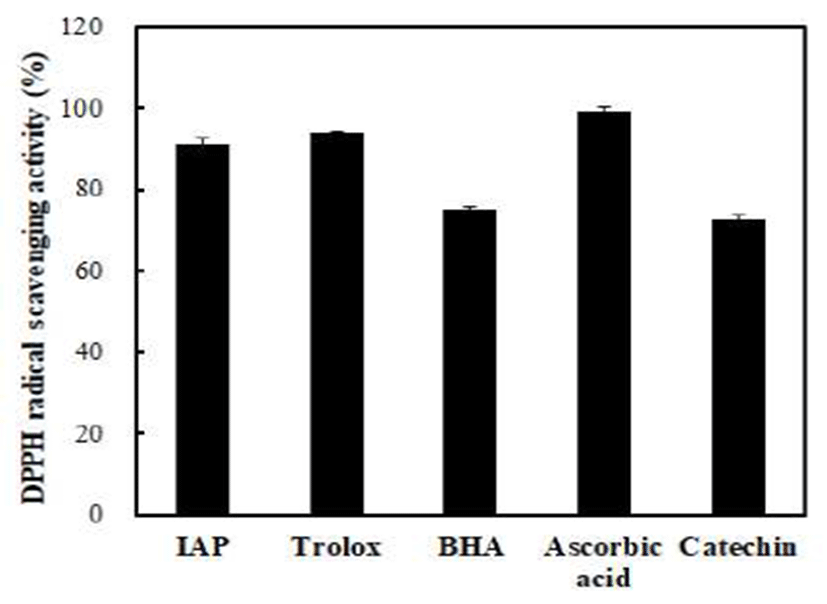
To evaluate the antioxidant activity of IAP, DPPH radical scavenging activities of IAP and various positive controls were compared (Fig. 5). IAP effectively scavenged the DPPH radical. The DPPH radical scavenging activity of IAP was above 90% at a concentration of 1.0 mg/mL which was similar to that of the Trolox at a concentration of 0.1 mg/mL. Wang et al. (33) suggested that the DPPH method can be used for the primary characterization of the radical scavenging activity of bioactive compounds. In addition, Cheung et al. (34) reported that free radical scavenging such as DPPH radical scavenging is one of the well-known mechanisms caused through inhibition of lipid oxidation. Ranathunga et al. (35) indicated that high antioxidant activity induced by low molecular weight peptides may be caused by the ease of interaction between free radicals and low molecular weight peptides. Chen et al. (36) reported that antioxidant peptides with 5-16 amino acid residues could hinder the autoxidation of linoleic acid due to their remarkable ability to cross the intestinal wall and react more easily with free radicals. As a result, IAP showed potent the DPPH radical scavenging activity, which indicated that the IAP was good natural antioxidant compounds for the anti-lipid peroxidation. Therefore, we suggest that it is possible to produce natural antioxidants from A. japonicus roe proteins. However, it is necessary to do further study the structural characteristics of antioxidant peptide isolated from A. japonicus roe.