Introduction
Fresh fruits and vegetables are one of the important foods for health of consumers and quality of life. Consumers require safe fruits and vegetables having good quality, but food poisoning through consumption of fruits and vegetables is increasing. Numerous bacteria and virus which can cause diseases inhabit fresh vegetables, therefore it is essential to prevent fruits and vegetables from getting contaminated and further to remove microorganism which can cause such diseases before consumers consume the contaminated fruits and vegetables (1). However, there are no practical chemical and physical sterilization methods that remove all types of pathogenic microorganism from the surfaces or inside of fruits and vegetables without damaging the organoleptic properties or nutritional quality of fruits and vegetables.
Chlorine disinfectant is widely used due to its cost effectiveness and its sterilization effectiveness (2). Chlorine generally including Cl2, OCl- and HOCl, and HOCl has strongest sterilizing power (5,6). Chlorine disinfection has an variable effect on pathogenic microorganism of fruits and vegetables, but it is a useful method to prevent contamination caused by using microbiologically unsafe washing water (3). The effects of various disinfectants have been reviewed for an effect of reducing microorganism of fruits and vegetables, but no disinfectants can remove pathogenic microorganism
perfectly. In general, 50-200 ppm of sodium hypochlorite is used in processing fresh fruits and vegetables. However, it does not show a significant effect on reducing microorganism at a low concentration, but contaminates products at a high concentration, and sodium occasionally remains on the products (2,3,7). Generally, inactivation efficiency for microorganism by chlorine treatment varies according to temperature, pH contact time, contact efficiency, interfering substances, concentration of available chlorine. Chlorine shows the highest inactivation effect when there are no low pH, low turbidity and interfering substances (4).
Unlike the strong acidic hypochlorite water from electrolyzing sodium chloride, slightly acidic electrolyzed water (SAWE) is chlorinated sterilized water with active chlorine form of 20-30 ppm hypochlorous acid (HOCI) which is available chlorine concentration of slight acid (pH 6.0-6.5). Hypochlorous acid (HOCI) has the eighty fold sterilization effectiveness compared to hypochlorite ion at the same concentration. HOCl kills microbial cells by allowing glucose oxidation from chlorine-oxidizing sulfhydryl groups of a certain enzyme inhabit carbohydrate metabolism. Accordingly, it is expected that hypochlorous acid can be substituted for the existing sodium hypochlorite or strong acidic hypochlorite water as it can reduce harmfulness by Cl2 off-gassing or surface corrosion because it is relatively low in concentration (8-14).
Some studies report on the sterilization efficacy for the surface of the facilities used in vegetables, fruits, meat and food processing factories using various types of electrolyzed water including strong acid, weakly alkaline and neutral. Most of the experiments showed results on the comparisons of sterilizing power of different types of chlorine disinfectants or on the combination effect of temperature and different phytochemicals. However, in order to use electrolyzed water as a disinfectant in processing foods the reduction effect depending upon processing time, processing temperature or processing method is the essential requirement for design of the process. It is because of most available chlorine including HCIO has no selectivity in inactivation of microorganism, and its purpose is to minimize surface contamination of microorganism by physically inactivating using cleaning water in the washing process. Especially, in order to apply it to the industry, it is necessary to design an appropriate washing process. Various factors should be determined in order to design this process, but the most important factor is concentration and time within the range that does not decrease the quality of fruits or vegetables. This study was conducted to evaluate the sterilization efficiency necessary for process design by analyzing the features of sterilization for four types of vegetables using SlAEW which was made with dilute HCI.
Materials and Methods
Selected 4 vegetables (lettuce leaf, endive leaf, perilla leaf and kale leaf) commonly consumed were used for this experiment. Vegetable were purchased at a local market in Sungnam city and Seoul city, stored at 4±1°C, relative humidity (RH) 90-95% and used within 2 days.
SlAEW was generated by a flow type electrolysis apparatus (BC-240, Cosmic Round Korea Co., Seoul, Korea). The mechanism of producing SlAEW is described as follows: the apparatus electrolyzes diluted hydrochloric acid (6%, v/v) in a non-diaphragm electrolytic cell, and produces highly concentrated hypochlorous acid. This is then diluted with tap water for producing SlAEW. In the study, SlAEW was stored in polypropylene containers, and immediately used for the measurement.
A 50 g sample of each fresh vegetables was dipped for 30 min in SlAEW, and then all samples were drained for 10 min at room temperature. During dipping, polypropylene containers were used for each treatment. In this study, samples of fresh vegetables without dipping or chlorination were used as a control for total microbial count enumeration. The temperature of each solution for disinfection treatment was in the range of 20-22°C.
The pH was measured with a pH meter (model 520A, Orion research Inc., Indianapolis, MA, USA) using a pH combination electrode and oxidation-reduction potential (ORP) was measured with ORP meter (RM-12P, TOA Electronics Ltd., Tokyo, Japan) using an ORP electrode (PST-2019C). Available chlorine concentration of SlAEW was determined by spectrophotometric method using a spectrophotometer (DR/4000 V, HACH Co., Loveland, OH, USA) (15). The detection limit is 0.2 mg/L Cl2. Therefore samples were first diluted to desired lower levels of available chlorine concentration (ACC) using deionized water prior to measurement.
Standard enumeration methods were used to determine the microbial growth. Three random samples were taken on each evaluation time. To enumerate the microorganisms, 25 g of each sample was combined with 225 mL of sterile 0.85% (w/v) sodium chloride solution in a sterile polyethylene bag (Model 400 Bags 6141, London, UK), and homogenized with a stomacher (Seward Stomacher 400, Seward, UK) for 5 min at 230 rpm The aliquot was used for various serial dilutions. The total microbial counts were determined by pouring 1 mL of diluted sample onto sterilized plate count agar (Difco Laboratories, Inc., Detroit, MI, USA). The plates were incubated at 30°C for 48 h, and the colonies counted. The microorganism counts of fresh vegetable sample were expressed in log CFU/g.
The calculated mean surviving microbial population and microbial log reductions (log CFU/g) as the result of treatment using SlAEW were considered for further statistical analysis. The values reported for plate count are the mean values of 8 individual trials±SD for each vegetable sample. Data were subjected to one way analysis of variance (ANOVA) was used to determine the differences at ≤0.05 using SAS.
Results and Discussion
Fresh vegetable used in this study shows a difference of more than 2-3 log CFU/g in total microbial count because the degree of contamination varies according to producing areas or seasons (16). Considering this characteristic, four types of vegetables including leafy lettuce which was produced and distributed in summer and fall when the degree of contamination was high were used as sample for the experiment, and the deviation among the samples was 1-3 log CFU/g, which was the same as other research results. Total microbial count of 8 types of lettuces sold at the market was 6.0±0.6 log CFU/g on average, and the maximum value was 6.7 log CFU/g and the minimum value was 4.0 log CFU/g (Fig. 1).
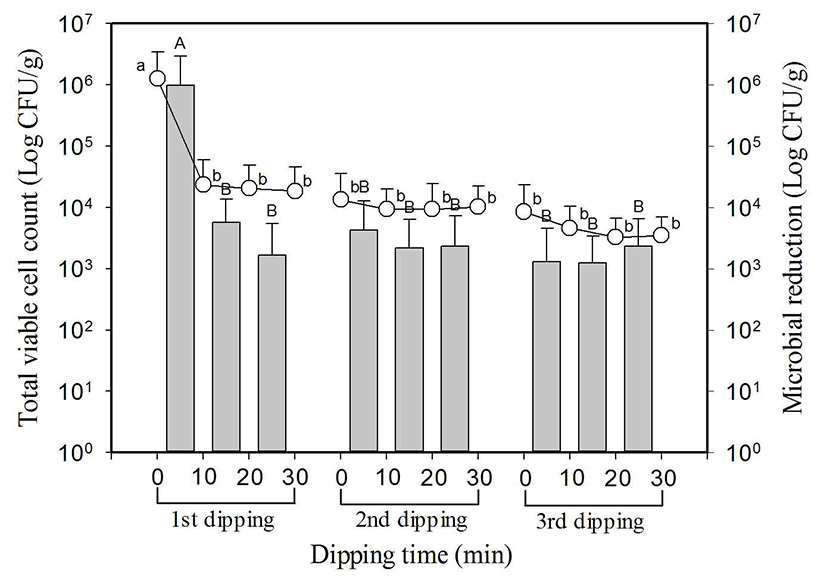
 , indicate reduced microbial population in each dipping stage.
SlAEW was with average 30 ppm of available chlorine, 562±23 of oxidation-reduction potential (ORP) and pH 6.4 at 20±1°C. Values with different letters (a, b, c, or A, B, C) in the figure are significantly different at p<0.05 by Duncan’s multiple range test. Data represent means of eight replications±SD.
, indicate reduced microbial population in each dipping stage.
SlAEW was with average 30 ppm of available chlorine, 562±23 of oxidation-reduction potential (ORP) and pH 6.4 at 20±1°C. Values with different letters (a, b, c, or A, B, C) in the figure are significantly different at p<0.05 by Duncan’s multiple range test. Data represent means of eight replications±SD.
Total microbial count decreased significantly (p<0.05) from 6.0±0.6 log CFU/g in the first dipping to 4.1±1.1 log CFU/g after 10 min, and it decreased by approximately 1 log CFU/g to 4.0±1.1 log CFU/g and 4.0±1.1 log CFU/g respectively after 20 min and 30 min, but there was no significant difference. In the second dipping and third dipping, there was no significant difference (p<0.05) as it was shown by 4.0±1.1 log CFU/g first to 3.8±1.0 log CFU/g after 30 min and 3.8±1.2 log CFU/g at initial to 3.6±0.5 log CFU/g after dipping for 30 min. Total microbial count reduced in three dipping was 2.2 log on average, and total microbial count reduced in the first dipping for 10 min was 1.7 log CFU/g. Most of the reduction happened within first 10 min.
Total microbial count of 8 types of perilla leaves was 4.8±0.4 log CFU/g on average, and the maximum value was 5.1 log CFU/g and the minimum value was 4.0 log CFU/g (Fig. 2). Total microbial count in the first dipping decreased significantly (p<0.05) from 4.8±0.4 log CFU/g at initial to 3.4±0.4 log CFU/g after 10 min, but there was no significant decrease as it showed 3.5±0.3 log CFU/g and 3.3±0.5 log CFU/g after 20 min and 30 min respectively. In the second dipping and third dipping, there was no significant difference (p<0.05) as it was shown by 3.3±0.4 log CFU/g first to 3.1±0.2 log CFU/g after 30 min, and 3.2±0.2 log CFU/g first to 3.0±0.2 log CFU/g after 30 min respectively. Total microbial count reduced from three time dipping was 1.8 log on average, and most of the reduction happened within first 10 min.
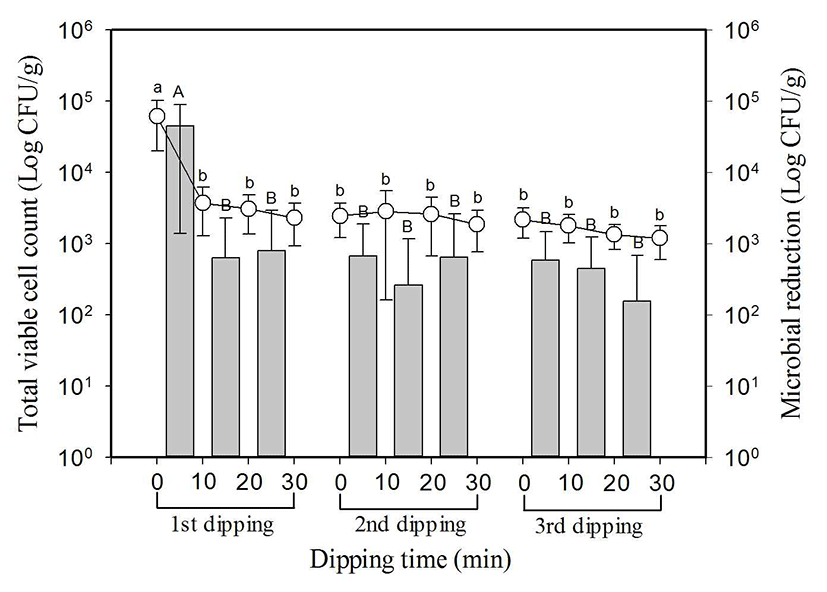
 , indicate reduced microbial population in each dipping stage.
SlAEW was with average 30 ppm of available chlorine, 562±23 of oxidation-reduction potential (ORP) and pH 6.4 at 20±1°C. Values with different letters (a, b, c, or A, B, C) in the figure are significantly different at p<0.05 by Duncan’s multiple range test. Data represent means of eight replications±SD.
, indicate reduced microbial population in each dipping stage.
SlAEW was with average 30 ppm of available chlorine, 562±23 of oxidation-reduction potential (ORP) and pH 6.4 at 20±1°C. Values with different letters (a, b, c, or A, B, C) in the figure are significantly different at p<0.05 by Duncan’s multiple range test. Data represent means of eight replications±SD.
Fig. 3 shows that with endive the reduction of total microbial count depending on the number of dipping and time is similar to leaf lettuce and perilla leaf. Total microbial count of 8 types of endive was 6.1±0.5 log CFU/g on average, and the maximum value was 6.7 log CFU/g, and the minimum value was 5.7 log CFU/g. Total microbial count in the first dipping decreased significantly (p<0.05) from 6.1±0.5 log CFU/g first to 4.8±0.1 log CFU/g after 10 min, but there was no significant reduction as it was shown by 4.5±0.3 log CFU/g and 4.4±0.4 log CFU/g after 20 min and 30 min respectively. In the second dipping and third dipping, there was no significant difference (p<0.05) as it was shown by 4.3±0.4 log CFU/g first to 3.9±0.6 log CFU/g after 30 min and 3.8±0.7 log CFU/g first to 3.2±0.2 log CFU/g after 30 min respectively. Total microbial count reduced from three time dipping was 2.9 log on average, and total microbial count reduced in the first dipping for 10 min was 1.3 log CFU/g on average. Likewise with leafy lettuce and perilla leaf, most of the reduction happened within the first 10 min.
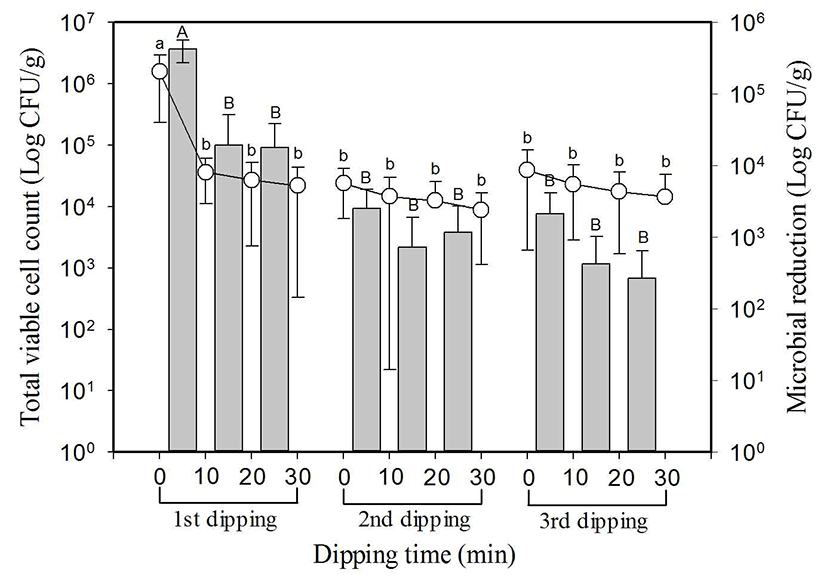
 , indicate reduced microbial population in each dipping stage.
SlAEW was with average 30 ppm of available chlorine, 562±23 of oxidation-reduction potential (ORP) and pH 6.4 at 20±1°C. Values with different letters (a, b, c, or A, B, C) in the figure are significantly different at p<0.05 by Duncan’s multiple range test. Data represent means of eight replications±SD.
, indicate reduced microbial population in each dipping stage.
SlAEW was with average 30 ppm of available chlorine, 562±23 of oxidation-reduction potential (ORP) and pH 6.4 at 20±1°C. Values with different letters (a, b, c, or A, B, C) in the figure are significantly different at p<0.05 by Duncan’s multiple range test. Data represent means of eight replications±SD.
Total microbial count of 8 types of kale leaf used in the experiment was 5.2±0.7 log CFU/g on average, showing the and the maximum value of 6.0 log CFU/g and the minimum value of 3.3 log CFU/g. Total microbial count in the first dipping decreased significantly (p<0.05) from 5.2±0.7 log CFU/g at initial to 3.5±1.2 log CFU/g after 10 min, but there was no significant reduction as it was shown by 3.4±0.9 log CFU/g and 3.5±1.0 log CFU/g after 20 min and 30 min respectively. In the second dipping and third dipping, there was no significant difference as it was shown by 3.6±0.9 log CFU/g at initial to 3.3±0.7 log CFU/g after 30 min and 3.4±0.5 log CFU/g at initial to 3.2±0.6 log CFU/g after dipping for 30 min, respectively. Total microbial count reduced from three time dipping was 1.6 log on average, and total microbial count reduced in the first dipping for 10 min was 1.3 log CFU/g on average. Likewise with leaf lettuce, perilla leaf and endive, most of the reduction happened within the first 10 min. Fig. 4.
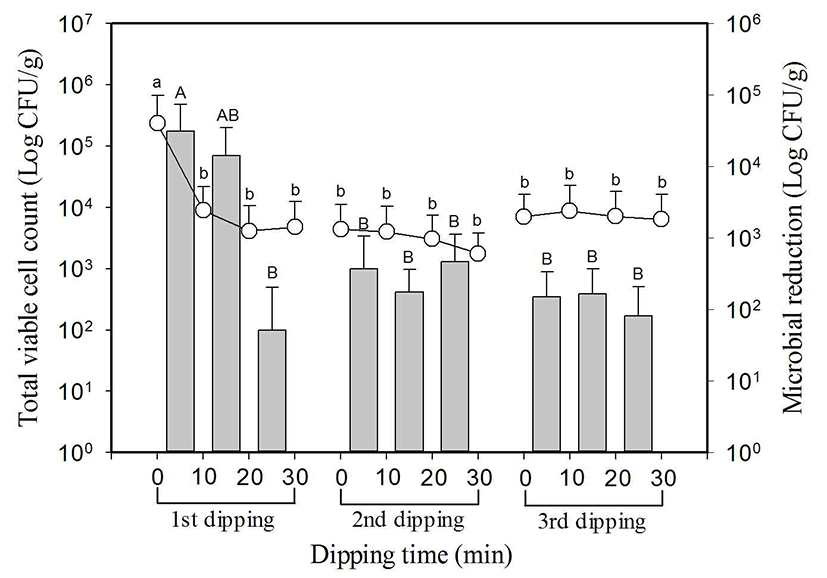
 , indicate reduced microbial population in each dipping stage.
SlAEW was with average 30 ppm of available chlorine, 562±23 of oxidation-reduction potential (ORP) and pH 6.4 at 20±1°C. Values with different letters (a, b, c, or A, B, C) in the figure are significantly different at p<0.05 by Duncan’s multiple range test. Data represent means of eight replications±SD.
, indicate reduced microbial population in each dipping stage.
SlAEW was with average 30 ppm of available chlorine, 562±23 of oxidation-reduction potential (ORP) and pH 6.4 at 20±1°C. Values with different letters (a, b, c, or A, B, C) in the figure are significantly different at p<0.05 by Duncan’s multiple range test. Data represent means of eight replications±SD.
Sterilizing effects of electrolyzed water showed great differences within range of 0.6-6.0 log CFU/g because the selected samples, types or concentration of electrolyzed water, and processing conditions for the samples tested by the researchers are all different (2,3). One of the most difficult problems in removing microorganism from vegetables and fruits using chemical disinfectant is to determine processing conditions such as concentration, time and temperature which can give an optimum sterilizing effect. SlAEW with 10 ppm of low concentration or SlAEW with 30-35 ppm directly decreased Escherichia coli (9), Vibrio parahaemolyticus (10), Bacillus cereus (11), Staphylococcus aureus et al. (12) by 6 log/mL. Amams et al. (17) reported surfactant significantly increased the reduction effect. No change in total microbial count after the first dipping for 10 min in this study due to limited contact between SlAEW and microorganism.
However, the sterilizing effect in the first dipping up to 10 min in this experiment seems to depend on concentration of chlorine and washing time as shown in Pirovani et al. (18). The recent studies explain that the reason why it is difficult to remove microorganism from fresh vegetables effectively using non-thermal technology such as the application of disinfectant is because of the formation of biofilm (19), internalization and infiltration (20,21). Estimates of biofilm abundance in phyllosphere communities show that bacteria in biofilms constitute 10-40% of the bacterial population on broad-leaf endive and parsley (22). Listeria monocytogenes, in a multispecies biofilm containing Pseudomonas fragi and Staphylococcus xylosus has been reported to be essentially unaffected by treatment with 500 ppm free chlorine (23). The formation of biofilms on leaf surfaces of spinach, lettuce, Chinese cabbage, celery, leek, basil, parsley and endive has been demonstrated (24). It is assumed that the ecological state of existing microorganism is an important factor which causes the difference of the reduction effect among the samples in this experiment. It is directly connected with the limited contact. From this perspective, according to the result of the examination of the correlation between total microbial count of vegetables before dipping and the survived population after 30 min, as shown in Fig. 5, the vegetables which had the same level of total microbial count showed a difference in sterilizing effects of maximum 2 log cycle, and the survived population did not decrease below 3 log CFU/g regardless of dipping time and number of dipping.
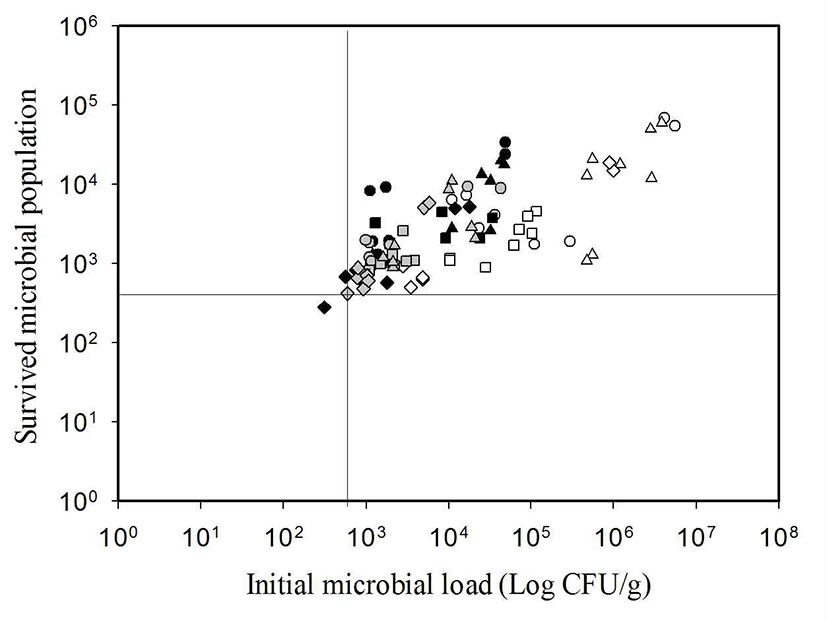
On the other hand, most of sterilizing effects happen in first 10 min of the first dipping, and microbial count reduced through the second dipping and third dipping was below 1 log cycle. It is assumed that it is difficult to expect an effective reduction effect after 10 min. Considering the results above, it is assumed that total microbial count of 3 log CFU/g which survived in the vegetables after dipping in disinfectant is the threshold that can reduce by dipping. Also, it is thought that microbial count which can be reduced by dipping in SlAEW for 10 min is approximately 2 log CFU/g on average. This result corresponds to the other studies (9,12-14). In order to increase the sterilizing effect, unless there is an excessive amount of microorganism, it is thought that applying the physical processing method which can increase the efficiency of contact can be an effective method than increasing the amount of available chlorine or processing time.
The reduction effect of available chlorine content tby dipping leafy lettuce, perilla leaf, endive and kale leaf in SlAEW through three steps was reviewed and the changes of available chlorine and pH were analyzed (Table 1 and Table 2). Available chlorine concentration was reduced in the first dipping by 3.2 ppm, 3.2 ppm, 3.4 ppm and 3.6 ppm units for leafy lettuce, perilla leaf, endive and kale leaf, respectively which corresponds a reduction of 10.6~12.1%.
However, available chlorine after first 10 min in leafy lettuce, perilla leaf, endive and kale leaf was 2.2, 2.0, 1.7 and 2.5 ppm respectively. It corresponds to 7.4%, 6.5%, 5.7% and 8.4% of the total amount of available chlorine respectively. Approximately 50-80% of the total reduction in available chlorine content disappeared in first 10 min. The amount of available chlorine reduced in the second dipping was 3.2, 3.1, 2.9 and 2.8 ppm for leafy lettuce, perilla leaf, endive and kale leaf respectively. The amount of the first available chlorine concentration was 10.7%, 10.4%, 9.5% and 9.2%, respectively. The amount of available chlorine which was similar to the first dipping disappeared on average. The amount of available chlorine reduced in the third dipping was 1.7, 2.5, 2.3 and 2.4 ppm for leafy lettuce, perilla leaf, endive and kale leaf respectively. Compared to the first dipping and second dipping, ratio of reduced concentration of available chlorine was 5.6%, 8.4%, 7.7% and 8.1%, respectively for the first available chlorine concentration.
pH changes tended to decrease generally according to the dipping time. In the first dipping, it decreased within the range of 0.09-0.12, and the value decreased at the initial treatment was 0.08, 0.05, 0.07 and 0.05 for leafy lettuce, perilla leaf, endive and kale leaf respectively. The fact that the range of pH value reduced in the second dipping was 0.05-0.11 and the range of value reduced in the third dipping was 0.06-0.07 was the same as the downward tendency of available chlorine.
The sterilizing power of electrolyzed water against microorganism has been sufficiently studied yet, but it is known that ORP and the relative concentration of chlorine species (aqueous molecular chlorine, Cl2, HOCl, OCl-) are the factors that are primarily connected with sterilizing power (4). Len et al. (5) proposed that hypochlorous acid (HClO) acts more efficiently on inactivation of microorganism than hypochlorite ion (OCl-). Hypochlorous acid as a weak acid has a strong oxidizing power, but it has an electrophilic characteristic, so it easily reacts with the ingredients of food and it enables oxidation, chlorine substitution or chlorine addition. If gaseous CI dissolves in water, the form of chlorine is changed according to the pH. Theoretically if hypochlorous acid takes more than 90% in pH 6, gaseous chlorine almost does not exist, but if pH increases, it dissociates itself into hypochlorite ion (3,7). In other words, the concentration of hydrogen ion in electrolyzed water has a direct effect on the HOCl/OCl- concentration ratio. This characteristic shows a distinct difference in sterilizing power in low concentration (8). However, most of vegetables are large in volume than heavy in weight, so it is assumed that the change of pH is unlikely to affect the sterilizing power because ten-fold SlAEW should be used for dipping based on the minimum weight ratio.
요 약
신선 농산물의 비가열 살균에 사용되는 살균소독제는 처리시간과 살균소독제의 농도에 비선형적인 감균효과를 나타낸다. 따라서 실제 사용에 있어서는 적정 농도와 적정 시간에 대한 고려가 매우 중요하다. 본 연구에서는 희석 염산(6%, v/v)을 원료로 생성한 미산성 차아염소산수 (slightly acidic electrolzyed water, SlAEW)(20±1°C에서의 유효염소 30 ppm, ORP 562±23 mV, pH 6.4)로 4종의 채소류 (상추, 깻잎, 치콘 및 케일)에 대한 미생물 저감 특성을 분석 하여 비가열 살균공정설계에 필요한 살균효과를 평가하였 다. SlAEW에 30분간 3회 침지하면서 핵심인자인 유효염소 와 미생물군수 및 잔류 미생물군수와의 관계를 분석하였 다. 대부분의 총균수 감소는 1차 침지 초기 10분간 이루어졌 으며 4종의 채소류에 생존하는 3 log CFU/g의 총균수가 침지를 통해 감소시킬 수 있는 한계값으로 판단되었다. 또 한 SlAEW에 10분간 침지함으로써 감소시킬 수 있는 균수 는 평균적으로 약 2 log CFU/g이었다. 초기 10분후의 감소 된 유효염소는 상추, 깻잎, 치콘 및 케일에 대해 각각 2.2 ppm, 2.0 ppm, 1.7 ppm 및 2.5 ppm이었고 감소된 유효염소 량의 약 50-80%가 초기 10분내에 감소되었다.