INTRODUCTION
Schisandra chinensis Baillon, is a deciduous woody vine that is native to forests in eastern Asian countries such as in northwestern China, far-eastern Russia, Korea and Japan. The deep red colored berries have five distinct tastes; sourness, sweetness, bitterness, saltiness and pungency. Because of the five distinct tastes, S. chinensis is known as “Omija” in Korean and “wu wei zi” in Chinese. The berries are used in traditional Chinese medicine and act as an astringent for the lungs and kidneys, to generate body fluid, and to reduce thirst (1). In Russia, S. chinensis has been used as an adaptogen to increases physical working capacity and protect against a broad spectrum of harmful factors such as heat shock, skin burn, cooling, frostbite, aseptic inflammation, irradiation and heavy metal intoxication (2).
The active ingredients of S. chinensis are lignans such as schisandrins and gomisins, which have been isolated and quantified from the seeds (3,4). Various biological activities of lignans from Omija berries have been investigated such as anti-oxidative protection (5,6), improvement of circulation and blood flow for cardiovascular disorder (7), enhancement in memory and learning (8), and hypnotic and sedative effect (9). However, the active ingredients in ethanolic extracts from the air-dried fruit or seed of S. chinensis appear to be fat-soluble which limit their bioavailability (10). On the other hand, the color of Omija fruit derived from anthocyans and flavonols has been identified and characterized (11,12,13). Water-based extracts from the fruit of S. chinensis also exhibit a wide range of biological functions such as anti-oxidative activities (12,13,14), antimicrobial activity (15), alcohol metabolizing activity (16) and stress treatment (17).
Nowadays, the dried fruit of S. chinensis has been commercialized as Omija-tea worldwide. In Korea, beverages such as Omija-cheong and Omija wines with fresh and/or dried fruits of S. chinensis are popular. Omija-cheong, known as a natural extract of S. chinensis, is produced in traditional manner, and is used for cold drinks and tea by diluting with drinking water. In order to produce Omija-cheong in traditional manner, equal quantities of fresh Omija fruits and sucrose are mixed and incubated at around 15℃ for 1 to 6 months. The low temperature incubation was conventionally used to prevent microbial spoilage and undesired fermentation (18). However, there is little information on the quality characteristics of Omija-cheong as a commercialized beverage in Korea. The aim of this study was to establish the optimal incubation time for manufacturing Omija-cheong by pilot-scale in Korean traditional manner, based on the physicochemical characteristics of Omija-cheong as a concentrated extract.
Materials and methods
Fresh Omija fruits were provided by Jangsu County (Jeonbuk, Korea), which were cultivated in Jangsu County and harvested in 2014. Food-grade white sucrose was supplied from CJ CheilJedang (Seoul, Korea). Reference standards of fructose, glucose, sucrose, maltose, oxalic acid, citric acid, malic acid, succinic acid, and gallic acid were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA), and quercetin was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). Folin-Ciocalteu’s phenol reagent was purchased from Sigma-Aldrich Chemical Co. The solvents for high-performance liquid chromatography (HPLC) and other chemicals were analytical grade reagents. Manufacturing equipment for Omija-cheong such as a bubble washer, a conveyor with air blower, 1,000 kg-scale incubation tanks with temperature control (Motiontec, Daejeon, Korea) were kindly provided by the Jangsu Center for Agricultural Technology (Jeonbuk, Korea).
The harvested fresh fruits were washed with tap and deionized water, blended with an electrical blender, and then used as crude samples. The crude samples were centrifuged and the supernatant were used as crude extracts for analyzing physicochemical characteristics. Soluble extracts were prepared by first removing the stems and seeds from washed Omija fruits manually, and 3 g of the flesh was soaked in 3 mL of deionized water, and sonicated for 30 min. Insoluble materials were removed by centrifugation and the supernatant was filtered for preparing soluble extracts. The soluble extracts of Omija fruits were used for determining the compositions of free sugars and organic acids using HPLC.
Fresh Omija fruits were washed with tap water using a bubble washer (Samjin Plant, Gyeongi, Korea). Excess water was drained and dried using the mesh-belt conveyor with an air blower. The washed Omija fruits (500 kg) were weighed and put into a 1,000 kg-scale incubation tank alternating with sucrose (500 kg) in equal quantities. For this study, we used a water-jacketed type incubation tank for a temperature control and an agitation system for intermittent mixing. The temperature of the incubation tanks was maintained at 15℃. The samples collected from the incubation tank were centrifuged to remove insoluble materials and used as concentrated extracts.
Moisture content of Omija fruits was measured using a moisture analyzer (ML-50, AND Co., Ltd., Tokyo, Japan). The soluble extracts and concentrated extracts were diluted 5 times with deionized water and pH levels were measured using a pH meter (Orion Star A211, Thermo Scientific, Waltham, MA, USA). Measurement of total sugar content (°Brix) was carried out with soluble extracts and concentrated extracts by using a refractometer with an accuracy of ±0.2 °Brix (PAL-1, ATAGO, Tokyo, Japan). According to the detection range of the refractometer, the concentrated extracts were diluted 2 times with deionized water. For measuring titratable acidity, the samples were diluted 10 times with deionized water and followed by titration with 0.1 N sodium hydroxide, using phenolphthalein as an end-point indicator. Titratable acidity was calculated and expressed in % (w/v) of acetic acid.
The color and color difference were analyzed with a Chroma Meter (CM-5, Konica Minolta, Tokyo, Japan) using the Hunter system, which identifies color using three attributes: L (white=100, black=0), a (red=positive, green= negative), and b (yellow=positive, blue=negative). The color difference (ΔE), a measure of the distance in color space between two colors, was determined by comparison to a deionized water with colorimeter values of L=99.98, a=-0.03, and b=0.01, using the following relationship:
For quantification of free sugar, the HPLC (Waters, Milford, MA, USA) system combined with a 410 differential refractometer was used with a LC-NH2 column (Supercosil™, 4.6 Φ×250 mm, Supelco, Bellefonte, PA, USA). The solvent system, consisting of an 80:20 acetonitrile to water ratio, was run isocratically at a flow rate of 0.8 mL/min. Fructose, glucose, sucrose and maltose were used for establishing standard curves. For analyzing organic acids, the HPLC system combined with a 486 Tunable absorbance detector (λ=210 nm) was used with a HPX-87H column (Aminex®, 7.8 Φ×300 mm, Bio-Rad Lab, Richmond, CA, USA). The solvent system, consisting of 0.01 M sulfuric acid, was run isocratically at a flow rate of 0.4 mL/min. Citric, oxalic, malic, succinic and acetic acid were used for establishing standard curves. The soluble extracts and concentrated extracts were injected to a HPLC system after filtration with a 0.45 μm membrane filter (Millipore Co., Billerica, MA, USA).
Total flavonoid contents of Omija fruits and Omija-cheong were determined using an aluminum nitrate colorimetric method (19) with minor modifications. An aliquot of the sample diluted with deionized water (0.5 mL) was mixed with 0.75 mL of 95% of ethyl alcohol and 0.75 mL of deionized water. Then 0.1 mL of 10% aluminum nitrate, 0.1 mL of 1 M potassium acetate, and 2.8 mL of deionized water were added and mixed. After incubation for 40 min at room temperature, the absorbance at 415 nm was measured using a spectrophotometer (Optizen POP, Mecasys Co., Daejon, Korea). The standard curve was prepared with quercetin solutions at 0~300 mg/L in 95% ethyl alcohol, and total flavonoid values were expressed in terms of quercetin equivalent (QE).
Total phenolic compound contents of Omija fruits and Omija-cheong were determined by the Folin-Ciocalteu colorimetric method (20) with minor modifications. An aliquot of the sample diluted with deionized water (0.1 mL) was mixed with 0.5 mL of Folin-Ciocalteu’s phenol reagent and 6.0 mL of deionized water. After incubation for 2 min at room temperature, 2 mL of 15% sodium carbonate and 1.4 mL of deionized water were added and mixed. After incubation for 120 min at room temperature, the absorbance was measured at 755 nm. The calibration curve was prepared with 0~170 mg/L solutions of gallic acid in 95% ethyl alcohol, and total phenolic values were expressed in terms of gallic acid equivalent (GAE).
All experiments were performed in triplicates and mean values and standard deviations were reported. Analysis of variance was conducted and the mean separations were analyzed using the Duncan’s multiple range tests (p<0.05). The statistical analyses were conducted using the SPSS for Windows 12.0 software (SPSS Inc., Chicago, IL, USA).
Result and discussion
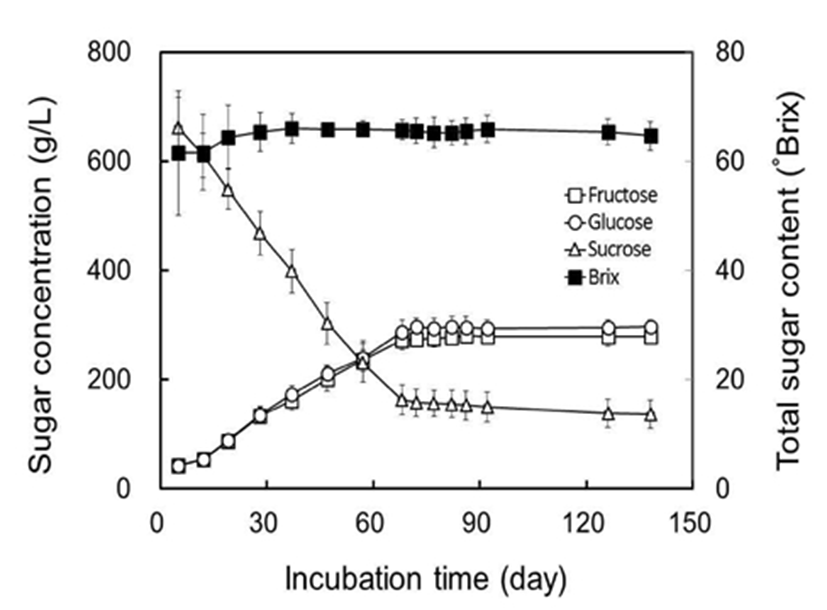
Moisture contents of fresh Omija fruits was measured to be 80.0%. Previous studies have reported moisture content of fresh fruits had in the range of 79.6% to 86.4% (21,22) which are similar with the results in this study. Total sugar content of crude extract from fresh Omija fruits was determined to 9.7 °Brix, which was slightly lower than previously reported (22). As shown in Fig. 1, total sugar contents of Omija-cheong ranged from 61.5 to 65.3 °Brix during 28 days of incubation, and stabilized at around 66.0 °Brix after 37 days of incubation. Based on a traditional manner, equal quantities of fresh Omija fruits (500 kg) and sucrose (500 kg) were alternatively mixed in 1,000 kg-scale incubation tank for preparing Omija-cheong. High content of sucrose in processing Omija-cheong at low temperature needs time to not only solubilize sucrose but also extract the soluble portions of Omija fruits by osmotic pressure of sucrose. The status of sucrose at initial stage in processing Omija-cheong was a major limit in mixing, and caused the fluctuation in total sugar content. The composition of free sugars in Omija fruits were determined to 18.5 g/L of fructose, 16.6 g/L of glucose, and 15.1 g/L of sucrose by using soluble extracts. The major sugar components of fresh fruits harvested in 2005 from Jangsu County (Korea) were well matched with the results of our study (23). Decreases in sucrose content with increases in both fructose and glucose contents in fresh Omija fruits also have been previously reported, which depend on the postharvest ripening conditions (23). The concentration of supplemented sucrose decreased with increases in both fructose and glucose according to the incubation time in processing Omija-cheong (Fig. 1). In our study, the conversion of sucrose into glucose and fructose continued to 68 days of incubation, and was minimized after 68 days of incubation. The composition of free sugars in Omija-cheong was maintained until 138 days of incubation, and almost 80% of supplemented sucrose was converted into glucose and fructose. Changes of sugar composition in fermented Omija with sucrose have been reported, where 50.3% of the supplemented sucrose was exhausted and has been converted into 22.9% of glucose and 21.5% of fructose during 6 months of fermentation (24). Based on the changes of free sugars in manufacturing Omija-cheong, it requires at least 68 days of incubation at a given condition.
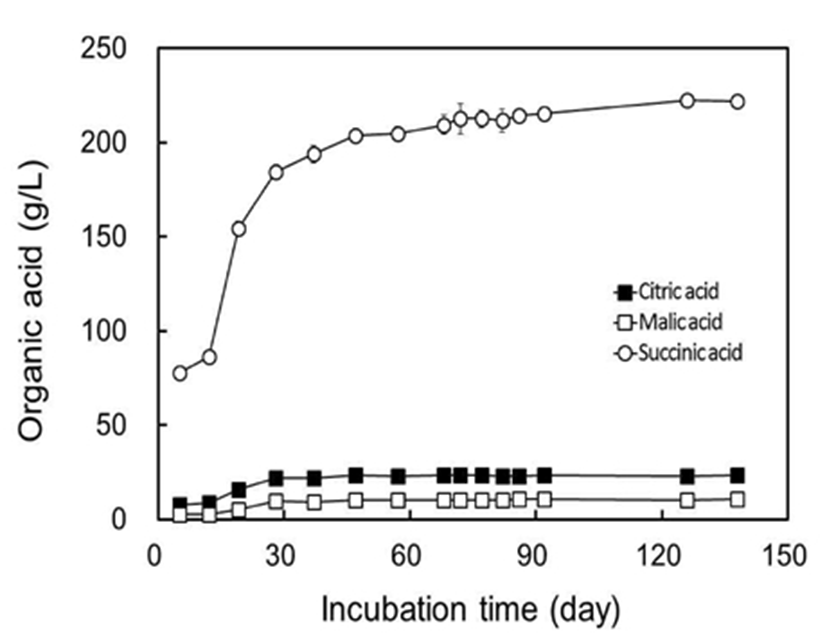
pH, titratable acidity, and composition of organic acids in Omija fruits and Omija-cheong are summarized in Table 1. The pH levels of Omija fruits was 3.07, which was slightly higher than reported in previous studies with fresh fruits (22). The pH of Omija fruits was also affected by postharvest conditions and gradually decreased from 2.81 to 2.68 according to the postharvest time (23). The pH level at initial stage (5 days of incubation) in processing Omija-cheong was 2.96 which slightly lower than that of Omija fruits, and slightly decreased to 2.90 at 92 days of incubation. Titratable acidity of fresh Omija fruits was determined to be 1.16% (w/v). According to the processing Omija-cheong, titratable acidities gradually increased from 1.18% (w/v) at 5 days, to 2.71% at 138 days of incubation. The organic acids in Omija fruits were 19.2 g/L of citric acid, 6.30 g/L of malic acid, and 159 g/L of succinic acid with the soluble extracts. Succinic acid was a major organic acid in this study, which is well matched with the previous report (23). The contents of organic acids were also affected by the postharvest ripening conditions, and the quantities of organic acids increased two-fold after 8 days of postharvest storage. On the other hand, fresh Omija fruit purchased from Hongcheon County (Korea) in 1998 showed a different composition of organic acids with 0.33% citric acid and 3.87% malic acid (25). Water-based extracts from dried Omija fruits contained 3.9~16.1% of citric acid as a major organic acid with 0.023~4.6% of succinic acid as a minor organic acid (12,25,26). The differences in composition of organic acids might be dependent on the varieties of S. chinensis, conditions of cultivation and postharvest, status of dehydration and experimental conditions. The changes of organic acids in concentrated extracts from processing Omija-cheong are shown in Fig. 2. Most of the citric, malic, and succinic acids in fresh Omija fruits were extracted into Omija-cheong rapidly within 37 days of incubation and reached to the stable concentrations after 47 days of incubation. The increase in titratable acidity was directly dependent on the contents of total organic acids in Omija-cheong during processing (r=0.97, p<0.01). Based on the changes of organic acids contents during Omija-cheong processing, it requires at least 47 days of incubation for constant quality at a given condition.
The Hunter index for color characteristics of Omija fruits and Omija-cheong were quantified by lightness (L), redness (a), yellowness (b), and summarized in Table 2. As a major color of Omija fruits with soluble extracts, the value of redness was 49.2, whereas the values of lightness and yellowness were 19.0 and 32.8, respectively. In processing Omija-cheong, the value of redness was around 68.0 until 19 days of incubation, and gradually decreased to 60.1 after 68 days of incubation. The Hunter index for lightness, redness, and yellowness were stabilized and maintained at around 43.0, 60.1, and 25.9 after 68 days of incubation, respectively. The variation in Hunter index at early stage of Omija-cheong processing might be related to the status of sucrose which was not dissolved yet. The colors of anthocyanins from S. chinensis fruits were reported to be depended on both thermal degradation and ultraviolet radiation (13,27). However, the Hunter index of Omija-cheong in this study was remained to be constant because the incubation was carried out with stainless tank which prevents sun-light. Based on the color characteristics, it requires more than 68 days of incubation for Omija-cheong manufacture at a given condition.
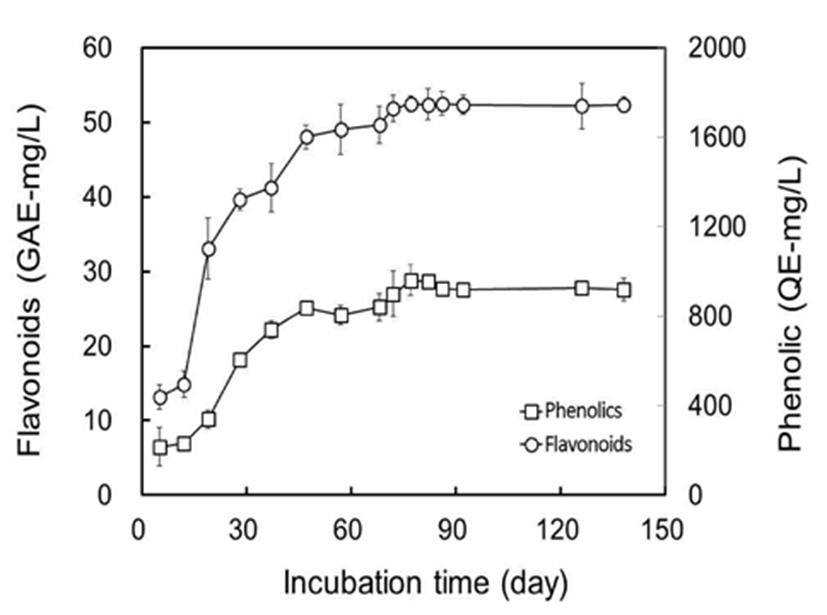
As bioactive ingredients, total flavonoids and phenolic compounds in Omija fruits and Omija-cheong were analyzed with gallic acid and quercetin as standards, and are shown in Fig. 3. The flavonoid concentration in crude extracts from Omija fruits was quantified to be 2.91 mg-GAE/L. The concentration of flavonoids in Omija-cheong was 6.50 mg-GAE/L at 5 days of incubation, and dramatically increased to 22.2 mg-GAE/L at 37 days of incubation. The concentration of flavonoids in Omija-cheong was maintained at around 24.1 mg-GAE/L after 57 days of incubation. The content of flavonoids in Omija-cheong was more than 8 times higher than that in Omija fruits. The concentration of phenolic compounds was 297 mg-QE/L in Omija fruits. The concentration of phenolic compounds in Omija-cheong was 439 mg-QE/L at 5 days of incubation, and increased to 1,322 mg-QE/L at 28 days of incubation. The concentration of phenolic compounds in Omija-cheong was maintained at around 1,635 mg-QE/L after 57 days of incubation. The content of phenolic compounds in Omija-cheong was more than 5 times higher than that in Omija fruits. The increases in both flavonoids and phenolic compounds during Omija-cheong processing might be due to the extended incubation time with sucrose. The wide ranges in the contents of flavonoids and phenolic compounds depended on extraction methods with solvents from S. chinensis (13,28,29,30). The changes of phenolic compounds showed a similar profile as that of flavonoids during Omija-cheong processing (Fig. 3). Based on the changes of flavonoids and phenolic compounds in manufacturing Omija-cheong, it requires at least 57 days of incubation for stabilized concentrations at a given condition.
요 약
전통적인 저온숙성 당절임 방법을 이용한 오미자청의 제조과정에서 최적의 배양시간을 찾기 위하여 파일럿 규모 (1톤)의 연구를 시행하였다. 생오미자와 동일한 량으로 투 입된 설탕은 배양시간에 따라 과당과 포도당으로 전환되었 으며 오미자청에 존재하는 유리당의 조성은 68일 이상의 배양기간에서는 일정하게 유지되었다. 배양기간에 따른 오 미자청의 pH는 일정한 수준을 유지한 반면, 적정 산도와 유기산의 함량은 배양시간 37일까지 지속적으로 증가한 이후 68일 이후에는 일정한 조성을 유지하였다. 생오미자 에 존재하는 주요 유기산은 숙신산으로 확인되었으며 오미자청에 존재하는 유기산의 조성 역시 생오미자와 유사하였 다. 오미자에 존재하는 주요 생리활성물질인 총 플라보노 이드와 폴리페놀화합물은 60일 이상 배양한 오미자청에서 높은 함량을 보이고 138일까지 일정한 수준을 유지하였다. 특히, 오미자청에 함유된 총 플라보노이드와 폴리페놀화합 물의 함량은 생오미자 과육에 비하여 각각 9배와 5배 정도 높은 수준으로 증가하였다. 생오미자를 저온숙성 당절임 방법을 적용하여 오미자청으로 제조·가공하는 과정에서 유리당, 유기산 및 생리활성물질 등에 대한 일정한 품질을 확보를 위해서는 최소 60일 이상의 배양기간이 필요한 것 으로 확인되었다. 이러한 연구결과는 전통적인 저온숙성 당절임 방법으로 제조·가공되는 오미자청의 고품질화와 기능성 음료 개발을 위한 제조·가공공정의 표준화에 유용 한 정보가 될 것으로 판단된다.