Introduction
Similar traditional herbs have been used in Korea, China, and Japan due to their geographical, cultural, and political, and climatic connections (1). Among these, Polygonum multiflorum Thunberg is a popular traditional herbal medicine, which is called “Hasuo” in Korea (“Heshouwu” in China) (2,3). There are two kinds of “Hasuo”, “Jeok Hasuo” and “Baek Hasuo (Back Suo)”. Although the names are similar, the origin of the two plants and their botanical characteristics are different. Jeok Hasuo is a root tuber of Polygonum multiflorum Thunberg belonging to Polygonaceae family. Baek Hasuo is a root tuber of Cynanchi wilfordii Radix belonging to Asclepiadaceae family (2,3). The difference of botanical characteristics between the two plants lies in the color; Jeok Hasuo is reddish brown, and Baek Hasuo is yellowish brown. In addition, Baek Hasuo contains Baekmilky liquid that is easily differentiated from Jeok Hasuo (4).
In medicinal studies, Hasuo has been used to treat age-related diseases and for suffering baldness and hair loss (5). In these studies, Hasuo exhibited the various functional properties including blood cholesterol-lowering activity, anti-oxidative activity, anti-inflammatory activity, anti-cancer activity, and neuroprotective effect against ischemia/ reperfusion injury (5,6). The major functional components of Baek Hasuo are phosphatidyl choline, phosphatidyl ethanolamine, phosphatidyl inositol, sarcostin, deacylnanchogenin, deacylmetaplexigenin, hidjoranin, caudatin, penupogenin, methyleugenol, daucosterol, acetovanillone, sucrose, geniposide, and succinic aicd (1,7). The major functional components of Jeok Hasuo are emodin, chrysophanol, rhein, physcion, tetrahydroxystilbene, glucopyranoside, monogalloyl ester, resveratrol, polydatin, rhaponticoside, polygonumosides, tricin, rutin, luteolin, quercetin, kaempferol, apigenin, and schizandrin (1,7).
Compared to the extensive research on antioxidant activity of Hasuo, research on antimicrobial activities of Hasuo is limited. Naturally occurring antimicrobial compounds in traditional herbs play an important role in the natural defense or competition systems of living organisms including microorganisms, insects, animals and plants (8). Since Hasuo contains flavones and flavonoid glycosides that may contribute to antimicrobial activity, it can be hypothesized that Hasuo exhibits antimicrobial activity. The pathogen Staphylococcus aureus has been a concern not only in food products but also in human skins, because S. aureus is one of common pathogens that live on the skin and mucous membranes of humans. In addition, Propionibacterium acnes has been known to be one of the major pathogens associated with acnes. Therefore, the main objective of this research was to determine and compare the antioxidant and antimicrobial activities of selected Jeok Hasuo and Baek Hasuo root extracts against skin-related pathogenic bacteria.
Materials and Methods
Dried roots of Jeok Hasuo (Polygonum multiflorum Thunberg, PM) and Baek Hasuo (Cynanchi wilfordii Radix, CM) were purchased from a local farm located in Cheonan, South Korea harvested in June 2016. The purchased PM and CW were kept frozen at -20℃ prior to extraction. Gallic acid and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Propionibacterium acnes KCTC 3314 and Staphylococcus aureus KCTC 3881 were used in this study. P. acnes and S. aureus were cultured in reinforced clostridial medium (RCM, Difco Laboratories, Detroit, MI, USA) and nutrient broth (Difco Laboratories, Detroit, MI, USA), respectively. P. acnes culture was incubated at 37℃ for 72 hr under anaerobic conditions in a candle gas jar (GasPak EZ Container Systems, Franklin Lankes, NJ, USA). S. aureus culture was incubated in an incubation shaker at 37℃ with a 200-rpm shaking for 24 hr. All other reagents and solvents (Sigma Chemical Co., St. Louis, MO, USA) used in this study were analytical grades.
The dried roots of PM and CW were cut into small pieces and ground into fine powders using a grinder prior to extraction. Fifty grams of CM or CW were added in 500 mL distilled water, 80% ethanol, and butanol using a soxhlet extractor for 12 hr. After extraction, each extract was filtered through Whatman No. 2 filter paper and the extraction process was repeated twice (9). Then, the solvents of each extract were removed completely using a rotary evaporator (Rikakikal Co., Kyoto, Japan). The extracts were freeze-dried and stored at -20℃ prior to use. The extraction yield of the sample was determined using the following equation; Extraction yield (%) = [weight of extract (g)/weight of dried Hasuo (g)]×100. Proximate analysis of Hasuo root extracts was conducted following the standard AOAC method (10).
The total phenolic contents of Hasuo root extracts were determined following the Folin-Ciocalteu method with minor modifications (11). A portion of 0.50 mL of the diluted extracts was mixed with 0.25 mL Folin-Ciocalteu reagent, followed by the addition of 1.25 mL sodium carbonate (20% aqueous solution). The mixture was kept in the dark for 40 min, and the absorbance was measured at 725 nm using a spectrophotometer (UVmini 1240, Shimadzu, Kyoto, Japan). A standard curve was obtained using gallic acid with selected concentrations of 0.0, 0.02, 0.04, 0.06, 0.08, and 0.1 mg/mL. The total phenolic content of the extracts was expressed as gallic acid equivalents (GAE), which represented the phonolic content as the amount of gallic acid (mg) in 1.0 g sample.
An aliquot of 1.0 mL of 0.1 mM DPPH radical solution was dissolved in methanol and mixed with 0.5 mL of each Hasuo extract with a concentration of 0.05 mg/mL or blank methanol for negative control. The reaction solution was mixed, and the absorbance was recorded at 520 nm using a spectrophotometer. Gallic acid (1.5 mg/mL) was used as a standard. The DPPH radical-scavenging activity (%) was calculated by the following equation:
The scavenging activity of sample was also expressed as 50% effective concentration (EC50), which represented the concentration of sample exhibiting 50% DPPH radical scavenging activity (11).
The bacterial culture was diluted in the designated broth to contain 8.0 log CFU/mL. The PM and CW root extracts were also diluted with each broth to obtain the concentrations of 0.0, 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL in the presence of 0.1% Tween 60. The diluted extracts were filtered with sterilized filter (pore size 0.45 μm) before being added to the bacterial culture. An aliquot of 120 μL bacterial culture and 120 μL diluted extracts was incorporated into microplate wells (UV Star, Greiner bio-one, Frickenhausen, Germany). The absorbance was read at 620 nm immediately using a plate reader (Bio-Tek Instrumenting Co., Winooski, VT, USA). The microplate was then incubated according to the cultivation methods described above, and the absorbance was read at 620 nm again after incubation. A minimum inhibition concentration (MIC) at 24 hr was defined as the lowest concentration of antimicrobial that exhibited a complete growth inhibition. The growth inhibition was defined as less than 0.05 absorbance difference (the absorbance of the microplate wells at 24 hr minus the absorbance of the microplate wells at 0 hr) (11).
P. acnes and S. aureus were cultivated to possess the concentrations of approximately 106 CFU/mL. One milliliter of each culture was spread on RCM plates for P. acnes and on nutrient agar plates for S. aureus. Blank paper discs of 6 mm in diameter (BBL, Cockeysville, MD, USA) were impregnated with the selected concentrations of Hasuo root extracts (2, 5, and 10 mg/disc) and placed on the surface of the agar plates. The discs impregnated with Hasuo root extracts were placed under hood to evaporate ethanol and butanol prior to application on the agar plates. The plate containing P. acnes was incubated at 37℃ for 72 hr under anaerobic conditions, and the plate containing S. aureus was incubated at 37℃ for 24 hr. Streptomycin (5 mg/mL) (Dufhefa, Biochemie, Holland) and phosphate-buffered saline (PBS, pH 7.2) were used for positive control and negative control, respectively. After incubation, the diameter of inhibition zone was measured at two cross-sectional points and the average was taken to calculate the area of inhibition zone (12).
Each experiment was a completely randomized design. The entire analyses were repeated three times. The treatment factors were selected various solvents (DW, ethanol, and butanol) and two Hasuo root extracts (PM and CW). One way analysis of variance (ANOVA) was performed using SPSS software (version 11.5, SPSS Ins., Chicago, IL, USA). The differences among means were analyzed using Duncan, and the significance level was defined at p<0.05.
Results and Discussion
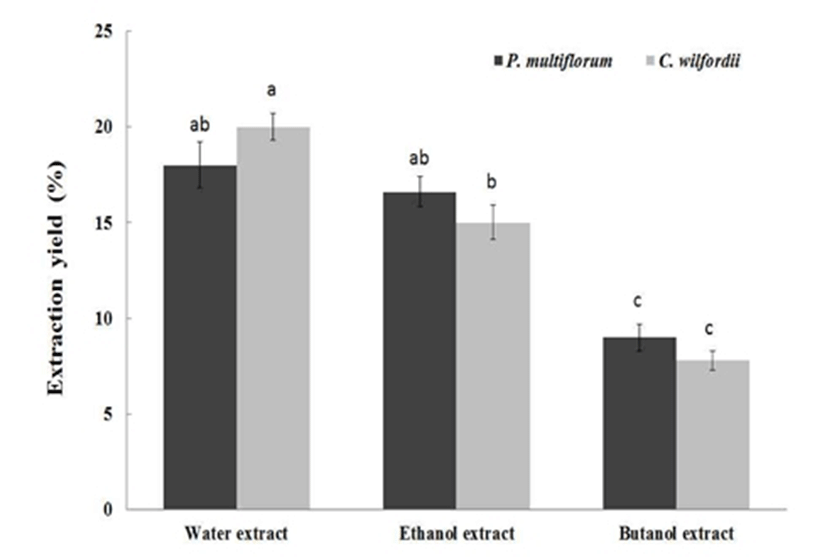
The proximate analysis of PM and CW roots are presented in Table 1. There was no significant difference in chemical compositions of PM and CW roots except for crude protein contents, however CW root contained a higher amount of crude protein than that of PM. When the Hasuo roots were extracted using different solvents, the water extract exhibited higher extraction yield than those of ethanol extract or butanol extract (Fig. 1). The butanol extracts of PM and CW exhibited the lowest extraction yields among the solvent extracts. However, there was no significant difference in extraction yields between PM and CW in the butanol extraction solvent. Meanwhile, the water extract showed the greatest extraction yield among the solvent extracts.
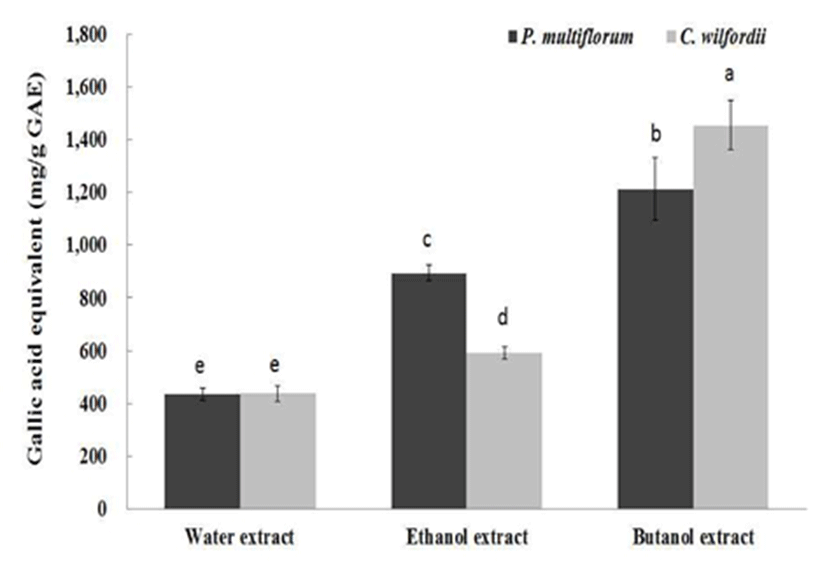
Phenolics or polyphenols have received growing attention because of recent findings on their biological activities (13). Most polyphenolic compounds are plant derived secondary metabolites and possess antioxidant activities, preventing lipid peroxidation, scavenging free radicals, and chelating redoxactive metal ions. Polyphenolic compounds include a wide range of chemicals including lavonoids and proanthocyanidins (14). The total phenolic contents expressed as gallic acid equivalent of PM and CW root extracted with selected solvents are presented in Fig. 2. The phenolic contents of PM and CW were significantly increased in the order of water extract, ethanol extract and butanol extract. The butanol extracts of PM and CW roots contained approximately 1,212.6 and 1,454.5 mg/g GAE, respectively. Wong et al. (15) also investigated the total polyphenolic contents of 60 Chinese medicinal plants, and the authors reported approximately 300 mg/g of total polyphenolic content of PM. The total phenolic contents of butanol extracts from PM and CW exhibited approximately three-fold greater phenolic contents than that of the water extracts. There was no distinct trend in the total phenolic contents between PM and CW.
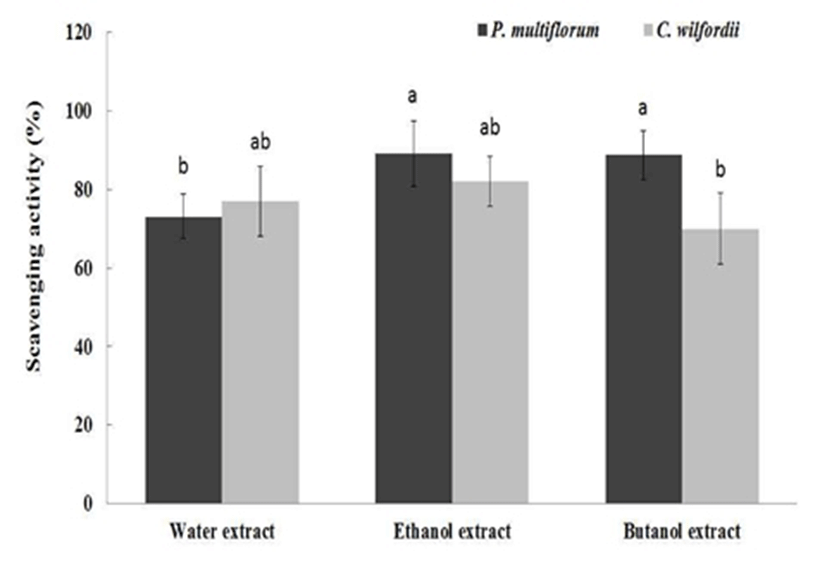
The DPPH scavenging activities of PM and CW root extracts with the concentration of 20 mg/mL are presented in Fig. 3. For PM, ethanol extract and butanol extract exhibited 89.0% and 88.8% scavenging activities, respectively, which were significantly higher than that of water extract (73.1%) (p<0.05). For CW, there was no significant difference among the extracts. It was well known that PM possessed strong antioxidant activities in vitro and in vivo (16,17,18). However, there is limited information on the functional activities other than antioxidant activities of Hasuo root extracts. In general, chemical compounds responsible for antioxidant activity also possess antimicrobial activities (19). Therefore, it can be anticipated that PM or CW root extracts might exhibit antimicrobial activities.
The minimum inhibitory concentrations (MICs) of PM and CW root extracts against P. acnes and S. aureus are presented in Table 2. The water extract and butanol extract of PM and CW did not exhibit any inhibitory effect against the tested bacteria at the tested concentration (1.0 mg/mL). However, the ethanol extracts of PM and CW exhibited MIC of 0.8 mg/mL and 1.0 mg/mL, respectively, against the tested bacteria. The results indicated that PM possessed higher inhibitory effects against tested bacteria than CW against tested bacteria. Although there is limited report on the antimicrobial activity of PM extract, there is no antimicrobial report on CW extract. Hasan et al. (20) investigated the antibacterial activity of Polygonum hydropiper root extract, similar to PM, and reported the MIC of 16 μg/mL against Staphylococcus aureus. The authors also indicated that the antibacterial potency of the plant extract expressed in MIC was stronger against gram-positive bacteria at lower concentration than it was against gram-negative bacteria.
The antimicrobial activities of PM and CW were also determined using a disc diffusion method, and the results are presented in Tables 3 and 4, respectively. Since the preliminary test using the similar ranges for MIC (up to 1.0 mg/disc) did not exhibit any zone of inhibition, the concentrations were increased to 2, 5 and 10 mg/disc. There was no formation of a zone of inhibition at 2 mg/discconcentration for all of the extracts. However, as the concentrations of extracts were increased, the zones of inhibition were formed, and the diameters of the zones were increased, which was proportional to the concentrations. The ethanol extract possessed the strongest inhibitory effects among the extracts, exhibiting the diameters of inhibition zone ranging from 8.9 to 9.2 mm against tested bacteria. The water extracts exhibited the weakest antimicrobial activities. There was no significant difference between the tested bacteria, presumably because the tested bacteria in this study are gram-positive bacteria.
Kwon (13) also confirmed the antimicrobial activity of PM against both Staphylococcus epidermidis and P. acnes. The authors could obtain the distinct inhibition zones from the water and 70% ethanol extracts. Hasan et al. (20) reported that chloroform extract of Polygonum hydropiper root (150 μg/disc) exhibited a 16-mm zone of inhibition against S. aureus. In good agreement with the previous reports, the PM and CW extracts possessed the antimicrobial activities against disease causing bacteria, especially for skin related diseases. S. aureus are a concern not only in food products abut also in human skins. S. aureus are a common type of pathogens that live on human skin and mucous membranes of humans. In addition, P. acnes are closely linked to the skin conditions such as acnes. Therefore, the PM or CW root extracts could be employed to develop skin-associated products and cosmetics due to the antimicrobial activity against skin-related bacteria.
요 약
본 논문은 국내 적하수오 및 백하수오 추출물의 항산화 능 및 항균활성을 구명하기 위한 목적으로 진행하였다. 각 하수오의 열수, 에탄올, 부탄올 추출물에 대하여, 일반성분 을 분석한 후, 항산화능에 대해서는 총 페놀함량 및 DPPH 라디칼 소거능을 측정하였으며, 항균활성에 대해서는 Staphylococcus aureus 및 Propionibacterium acne에 대한 최소생육저해농도(minimum inhibitory concentration, MIC) 및 생육저해환을 통하여 평가하였다. 적하수오 및 백하수 오 부탄올 추출물의 총 페놀함량은 각각 1,212.6 및 1,454.5 mg/g GAE로 다른 유기용매 추출물에 비해 유의적으로 높 은 함량을 보였다. 이에 비해 DPPH 라디칼 소거능은 열수 추출물에 비해 에탄올 추출물(89.0%), 부탄올 추출물 (88.9%)이 유의적으로 높은 값을 나타내었다. MIC 측정 결과, 적·백하수오 에탄올 추출물만이 0.8 mg/mL로 S. aureus와 P. acne에 대해서 항균효과를 나타냈다. 디스크 확산법 측정 결과, 모든 유기용매 추출물이 5 mg/disc 농도 부터 생육저해환을 형성하였으며, 농도가 증가함에 따라 생육저해환의 크기도 증가하였다. 적하수오 및 백하수오 에탄올 추출물의 조사균주에 대한 생육저해완의 직경은 10 mg/mL 농도에서 각각 8.9 및 9.2 mm를 나타내, 추출물 중 가장 우수한 항균활성을 나타냈다. 본 연구는 하수오 추출물이 항산화 효과 뿐만 아니라 피부 건강과 관련된 세균에 대해 항균활성을 보여줌으로써, 하수오 추출물이 피부 건강을 위한 천연 유래의 기능성 화장품 소재로도 활용될 수 있는 잠재적 가능성을 제시하였다.