Introduction
Reactive oxygen species (ROS), which include various free radicals (•OH, O2•− , NO•), hydrogen peroxide (H2O2), and singlet oxygen (1O2), are generated as a result of cellular metabolism through various pathways. Oxygen damage, caused by excess ROS, is believed to be deeply associated with inflammation, cancer, aging, cardiovascular diseases, and atherosclerosis (1,2). The internal antioxidant systems, which mainly consisted of superoxide dismutase, catalase, and glutathione, are not sufficient to restore the oxidative balance when the ROS accumulated largely. Therefore, for regulating the excess ROS, external antioxidants should be used. In recent years, a number of investigations have suggested that the natural antioxidants obtained from plants and other materials can help scavenge ROS, cause cardiovascular diseases to recede, and suppress inflammation (3-5).
Inflammation is an important process in the response to tissue damage stimulated by endogenous and exogenous factors. Mediators releasing during the process is a crucial part, which can affect the body’s defenses and decrease the potential damages (6). Nitric oxide (NO) is an important mediator which is produced from L-arginine, under the function of nitric oxide synthases (NOS). If it’s not suppressed in time, the accumulation of NO will promote an excess inflammation response, and even result in atherosclerosis, diabetes, Alzheimer's disease, asthma, cancer, and other diseases (7,8). Multiple lines of evidence have suggested that materials which exhibited potent inhibitory effect on inflammatory mediator production and synthases expression possess significant anti-inflammatory effect (9,10).
The Allium genus belongs to the larger Alliaceae family, which consists of more than 700 species. It is well known that the Allium species has been used as a flavoring ingredient for thousands years, and quite a few investigations have been conducted to evaluate their chemical characteristics and therapeutic effects (11,12). Among them, garlic (Allium sativum L.) and onion (Allium cepa L.) have been examined widely, and have demonstrated diverse biological qualities, including antioxidant, antihypertensive, and anti-inflammatory effects (5). As one member of the Allium genus, Allium hookeri (A. hookeri) is widely used as a food and a medical material in India, China, and other areas of Asia. Though some investigations on the bioactive properties of A. hookeri have been conducted previously, the works were mainly concerned with the antioxidant and anti-inflammatory activities, so the estimations on the bioactive components are likely inadequate (13-15). In this study, the antioxidant effects of the water, methanol, and ethanol extracts from the A. hookeri root were evaluated by various assays. The anti-inflammatory activity of the extracts was measured using RAW 264.7 macrophage cell lines. Furthermore, the total contents of three prime phytochemicals, polyphenols, flavonoids, and thiosulfinates were quantified.
Materials and Methods
The followings were purchased from Sigma Chemical Co. (St. Louis, MO, USA): Folin & Ciocalteu’s reagent, gallic acid, 1,1-diphenyl-2-picry-hydrazil (DPPH), rutin, L-ascorbic acid (VC), H2O2, sodium salicylate, butylated hydroxyanisole (BHA), sulfanilamide, N-(1-naphthyl)-3-thylenediamine dihydrochloride, 2,2′-Dithiobis (5-nitropyridine) (DTNB), β-Nicotinamide adenine dinucleotide (NADH), nitrotetrazolium blue chloride (NBT), phenazine methosulfate (PMS), dimethyl sulfoxide (DMSO), thiazolyl blue tetrazolium bromide (MTT), and lipopolysaccharide (LPS, from Escherichia coli O11:B4). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), and penicillinstreptomycin were obtained from Gibco BRL (Grand Island, NY, USA). Other chemicals and reagents used were of an analytical grade.
The A. hookeri root used in this study was obtained from the local market at Jeonnam, South Korea in January, 2014. A. hookeri root was washed with water to remove any contaminants, and dried at 45℃. Afterwards, the material was ground, using an electric grinder, into a fine powder. Ground powder of A. hookeri was stored at -20℃ prior to be used.
A water extract was prepared using 100 g of A. hookeri powder, homogenized with 500 mL of water and extracted at 121℃ for 1 hr (Autoclave, JSAC-100, JSR, Gongju, Korea). The filtrate was collected after 30 min of centrifugation (1,800×g), and filtration through a Whatman No.2 filter paper (Whatman, Maidstone, Kent, UK). The extraction process was done three times, and the collected filtrate was mixed. The filtrate was then evaporated under a vacuum and lyophilized. To compare the influence of different solvents on the extraction and physiological effects of the extracts, ethanol and methanol were also used to prepare samples simultaneously. The ethanol and methanol extracts were prepared using a 95% ethanol and 80% methanol aqueous solution (1:10, w/v), at 40℃ for 24 hr, with occasional stirring. This procedure was done three times, and the collected extracts were evaporated under a vacuum until they were dry. The acquired extracts were stored at -20℃ until further analysis.
The total phenolics content was determined using Folin-Ciocalteau’s reagent (16). The sample solution (100 μL) was mixed with 7.9 mL of distilled water and 0.5 mL of Folin-Ciocalteau’s reagent. After 15 min, 1.5 mL of 1.85 M sodium carbonate was added, and the mixture was maintained in the dark for 2 hr. The absorbance was measured at 765 nm by UV-visible spectrophotometer (Ultrospec 5300 pro, Biochrom Ltd., CB, UK) and total the phenolic content was calculated from the standard curve of gallic acid (y=1.299x+0.0074, R2=0.9996).
The total flavonoid content was measured according to the previous method (17). The examined sample (1 mL) was added to 4 mL of distilled water and 0.3 mL of 5% NaNO2 in a test tube. The mixture was allowed to stand for 5 min before 0.3 mL of 10% AlCl3 solution was added. After 6 min, 2 mL of 1 M NaOH was added. The final volume of the reaction mixture was increased to 10 mL with distilled water. The absorbance was read at 510 nm using rutin as a standard. Total flavonoid content was calculated from the standard curve of rutin (y=0.901x+0.0105, R2=0.9991).
The total thiosulfinate content was determined based on the method of Han et al. (18). An aliquot of sample was placed in a test tube, to which 0.5 mL of 2 mM cysteine was added. The total volume rose to 5 mL with hepes buffer (50 mM, pH 7.5). After incubating it at 27℃ for 15 min, 1 mL of the reaction solution was taken out and mixed with 1 mL of 0.4 mM DTNB. The mixture was incubated at 27℃ for 10 min and the absorbance was monitored at 412 nm. The total thiosulfinate content was calculated using equation (1):
where A0 represents the absorbance of cysteine without extract, and A1 is the absorbance of mixture containing examined extract. B is the dilution factor of cysteine.
The DPPH radical scavenging activity was evaluated using a method described by Yang et al. (19). Briefly, 2 mL of a 0.1 mM DPPH solution in 95% ethanol, was added to 2 mL of sample at various concentrations and allowed to stand in darkness for 30 min. The absorbance, at 517 nm, was read via a UV-visible spectrophotometer (Ultrospec 5300 pro, Biochrom Ltd., CB, UK). BHA was used as positive control and the scavenging effect was calculated according to equation (2):
where Acontrol is the absorbance of the control without the extract, and Asample is the absorbance of the mixture in the presence of extract.
The hydroxyl radical scavenging effect was measured using the method set forth by Smirnoff and Cumbes (20). In brief, 3 mL of the reaction mixture contained 0.3 mL of 20 mM sodium salicylate, 1 mL of 1.5 mM ferrous sulfate, 1 mL of the sample, and 0.7 mL of 6 mM H2O2. The mixture was incubated at 37℃ for 1 hr, and the final absorbance was measured at 562 nm. The scavenging capacity was calculated using formula (2). Vc was prepared in distilled water and used as positive control in current and following assays.
The scavenging effect of the superoxide radical was determined using a modified method described by Zhang et al. (21). Samples of 1.5 mL of each extract at different concentrations were mixed with 0.5 mL of the 468 μM NADH and 0.5 mL of the 300 μM NBT. After being shaken vigorously, 0.5 mL of the 60 μM PMS was added, and the reaction was kept at 25℃ for 5 min. The absorbance was read at 560 nm, and the scavenging effect was calculated according to equation (2).
The nitrite scavenging assay was performed using Griess reagent described elsewhere (22,23). The Griess reagent was a mixture of 0.1% of N-(1-naphthyl)-3-thylenediamine dihydrochloride and 1% sulfanilamide at a ratio of 1:1. Briefly, 1 mL of the sample was mixed with 1 mL of the 1 mM NaNO2, and then the pH was adjusted to 1.2 with the 0.1 M HCl. The incubation was carried out at 37℃ for 1 hr in water bath. A 1 mL portion of the mixture was then mixed with 2 mL of 2% acetic acid and 0.4 ml of the Griess reagent. The absorbance was read at 540 nm, after 15 min of being kept in darkened conditions, and results were calculated using formula (2).
The reducing power was assessed using according to the method of Yen and Chen (24). One millimeter of the sample was mixed with 2.5 mL of 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricynide. The mixture was incubated at 50℃ for 20 min. After being cooled to room temperature, 2.5 mL of 10% trichloroacetic acid was added, and the mixture was centrifuged at 3,000 rpm for 10 min. Thereafter, 2.5 mL of supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride solution. The results were expressed as the absorbance at 700 nm measured by spectrophotometer.
Murine RAW 264.7 macrophages (TIB-71) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were maintained in DMEM, supplied with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated FBS in a humidified incubator containing 95% air and 5% CO2 at 37℃.
MTT assay was performed to evaluate the cell viability in the presence of the samples. An aliquot of culture medium (200 μL), containing 1×105cells was seeded in a 96-well plate, and incubated at 37℃ for 24 hr. After the addition of the sample, the cells were incubated for another 24 hr in a CO2 incubator. Afterwards, the MTT solution (5 mg/mL) was added to each well and incubated for 4 hr. The formed purple formazan was dissolved by 100 μL of DMSO. Cell viability was determined by measuring the absorbance at 570 nm with microplate reader (μQuant, BioTek, VT, USA).
Nitrite is the stable product of NO in a cell culture medium, and it was measured using Griess reagent mentioned above. In brief, RAW 264.7 cells were cultured for 24 hr and then pre-treated with various concentrations of the sample. Thereafter, the LPS (2 μg/mL) was added to initiate NO generation. An aliquot of culture medium (100 μL) was removed, after 20 hr incubation, and mixed with 100 μL of Griess reagent. After 15 min, the absorbance of the mixture was read at 540 nm.
Statistical analysis was carried out using the SPSS statistical program (21.0, SPSS Inc., Chicago, IL, USA). The values were evaluated using one-way analysis of variance (ANOVA), followed by post-hoc Duncan’s multiple range tests. All data were presented as mean±standard deviation (SD) of multiple measurements. Differences were considered to be significant at the level of p<0.05.
Result and Discussion
Polyphenolic compounds are secondary metabolites with various chemical structures and characteristics. As presented in Table 1, the sample extracts contained different levels of total phenolics. The total phenolic content was found to be most abundant in the ethanol extract with the value of 24.94 mg gallic acid equivalents (GAE)/g extract. The water and methanol extracts contained less phenolic content, at 3.47 and 3.29 mg GAE/g extract, respectively. Though it was not reported in the methanol extract, the components in the ethanol and water extracts were found to comparable with one related study in which the total phenolic content was 21 and 6 mg GAE/g extract in the ethanol and water extracts, respectively (25). With respect to the total flavonoids, abundant content was achieved by ethanol and methanol. Among the extracts, the highest content, 4.27 mg rutin equivalents (RE)/g extract, was found in ethanol extract, while methanol extract contained relatively less content and water extract contained the lowest amount of total flavonoids (2.61 and 0.72 mg RE/g extract, respectively). However, the total flavonoid content in the study done by Lee et al. (25) was demonstrated to be undetectable. Therefore, the extraction conditions employed in this study were more effective in the preparation of flavonoid compounds from the A. hookeri root.
Previous study has indicated that organic solvents are more efficient than water in the extraction of phenolic compounds due to the less polarity and appropriate solubility (26). Moreover, the addition of water was demonstrated to increase the polarity of organic solvent and may explain the low amount of phenolic and flavonoid compounds in methanol extract (27). Relatively higher amount of total phenolic content in water extract may due to the high temperature used. Total phenolic and flavonoid contents obtained from A. ampeloprasum L. (methanol extract) were 5.70 mg GAE/g extract and 0.86 mg catechin equivalents/g extract (28). Lanfang et al. (29) reported that the total phenolic content of scallion prepared by water and 75% ethanol was varied from 8 to 12 mg/g dry weight. Thus, A. hookeri root can be recognized as a natural source of phenolic and flavonoid compounds.
For Allium genus, phenolic and flavonoid components were reported to contribute to the antioxidant and anti-inflammatory activities. The extracts obtained from different onion species were demonstrated to be rich in phenolic and flavonoid compounds (ferulic acid, vanillic acid, protocatechuic acid, isorhamnetin, quercetin, and kaempferol), and exhibited remarkable effects on the inhibition of lipid peroxidation and radical scavenging of DPPH, hydroxyl, and nitric oxide (30). Additionally, these extracts also exhibited satisfactory anti-inflammatory activity. With respect to flavonoid compounds, chrysoeriol-7-O-[2″-O-E-feruloyl]-β-d-glucoside, isorhamnetin-3-d-glucoside, and chrysoeriol, isolated from A. vineale, were reported to possess dominant antioxidant activity on the inhibition of lipid peroxidation, DPPH radical scavenging, metal-chelating, and reducing power (31). Total phenolics were indicated directly contribute to the antioxidant activity of scallion (29). Moreover, catechin, epicatechin, and taxifolin were found have efficient inhibitory effects on the NO and cytokines (TNF-α, IFN-γ) production, as well as the suppression of the NF-κB gene expression (10). Furthermore, the anti-inflammatory property of extracts from Securidaca longepedunculata root barks were found highly correlated with the total phenolic content (32).
The organic-sulfur compounds observed in the Allium genus consist mainly of flavor precursors, thiosulfinate compounds, and diverse derivatives. The common flavor precursors including alliin (S-alk(en)yl-L-cysteine sulfoxide), methiin (S-methyl systeine sulfoxide), propiin (S-propyl- L-cysteine sulfoxide), and isoalliin (S-1-propenyl cysteine sulfoxide), are present in the intact tissues. When the tissues are damaged, thiosulfinate compounds are formed under enzyme reaction and are responsible for the special flavor of the genus (12). Other sulfur constituents, such as diallyl, allyl methyl, and diethyl mono sulfides are derived from thiosulfinates under various conditions, and also contribute to the special flavor. It is well known that thiosulfinates are the most studied of the organosulfur components.
In the A. hookeri root, the major thiosulfinates observed included diallyl thiosulfinate (allicin, 56.6 μg/g of fresh weight), dimethyl thiosulfinate, allyl methyl thiosulfinate, and 1-propenyl methyl thiosulfinate (33). To our best knowledge, the total thiosulfinate content in the extracts of the A. hookeri root was reported for the first time. This assay is based on the reaction between cysteine and the functional group of -S(O)-S- of thiosulfinate compounds (18). Results indicated that largest content was in the ethanol extract (14.07 μM/g extract), and relatively low amounts were in the methanol and water extracts (6.35 and 2.52 μM/g extract, respectively). The amount of total thiosulfinates has been mentioned in other Allium species previously. Large amounts of total thiosulfinates were found in elephant garlic (A. ampeloprasum, 53 μM/g fresh weight) and wild garlic (A. ursinum, 21 μM/g fresh weight), whilst low contents were in Chinese chive (A. tuberosum, 2 μM/g fresh weight), various onions (A. cepa, 0.14-0.35 μM/g fresh weight), leek (A. porrum, 0.25 μM/g fresh weight), and scallion (A. fistulosum, 0.08 μM/g fresh weight) (11).
It was also reported that organic-sulfur compounds partially contributed to the antioxidant and anti-inflammatory effects of the Allium species. Allicin was found to be a strong scavenger against hydroxyl and DPPH radicals (34). Xiao and Parkin demonstrated that dimethyl thiosulfinate and dipropenyl thiosulfinate both possess strong hydroxyl radical scavenging activity, and weak effects on DPPH radical inhibition and signet oxygen quenching (35). Moreover, sulfur-containing compounds have shown effective antiinflammation properties. The anti-inflammatory activity of organic-sulfur compounds, especially thiosulfinates and their degradation products, has been described widely. The anti-inflammatroy effects of diallyl sulfide (DAS), diallyl disulfide (DADS), and allyl methyl sulfide have been evistigated, and these compounds caused reduction on the NO production in RAW 264.7 cells (36). Allicin and ajoene were indicated to significantly suppress iNOS mRNA expression (37).
The DPPH radical scavenging assay is widely employed to evaluate the electron or hydrogen donating ability of antioxidants due to its high degree of reliability, stability, and repeatability. As illustrated in Fig. 1, the extracts of A. hookeri root were capable of scavenging DPPH radicals in a concentration-dependent manner. The ethanol extract showed the strongest scavenging capacity with IC50 of 0.95 mg/mL (Table 2). The water and methanol extracts exhibited relatively lower effects with higher IC50 values of 3.43 and 6.57 mg/mL, respectively (p<0.05). Won et al. (38) reported that the 70% methanol extracts from the A. hookeri leaves and roots cultivated in different conditions, were able to scavenge the DPPH radicals and the IC50 values ranged from 2.74 to 8.74 mg/mL. Meanwhile, the crude ethanol extract of A. hookeri showed mediated DPPH radical scavenging activity and had an IC50 of 4.5 mg/mL (15). Therefore, the ethanol and water extracts possessed efficient scavenging activity, and the methanol extract also possessed mediated scavenging activity against the DPPH radical.
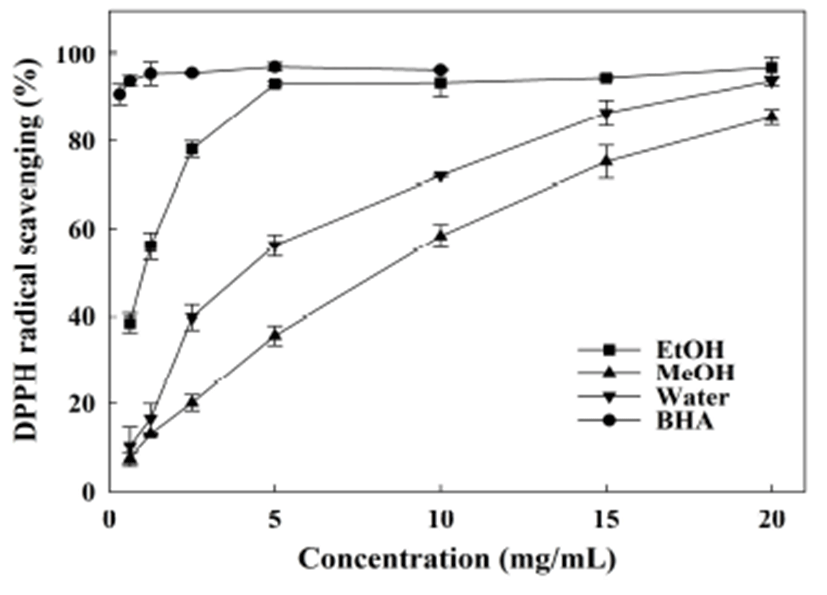
With excellent antioxidant activities were reported widely, high amount of phenolic, flavonoid, and thiosulfinate compounds may explain the strong DPPH radical scavenging activity of ethanol extract. For water extract, other compounds more than phenolic and thiosulfinate compounds may contribute the mediate antioxidant activity. Yin and Cheng indicated that phenolics and other compounds are more essential than allicin for the antioxidant activities of Chinese chive and Chinese leek (39). Futhermore, the thiosulfinates will degrade into various derivatives (e.g. diallyl, allyl methyl, and diethyl mono sulfides) under a variety of conditions including high temperature (12). Related research has indicated that DAS and DADS have the ability to attenuate lipid oxidation and also exhibit reducing power (40). Therefore, it is still unclear that which part of compounds play critical role for the antioxidant activity of water extract. With respect to methanol extract, the low total flavonoid and thiosulfinate compounds may explain the weak effect.
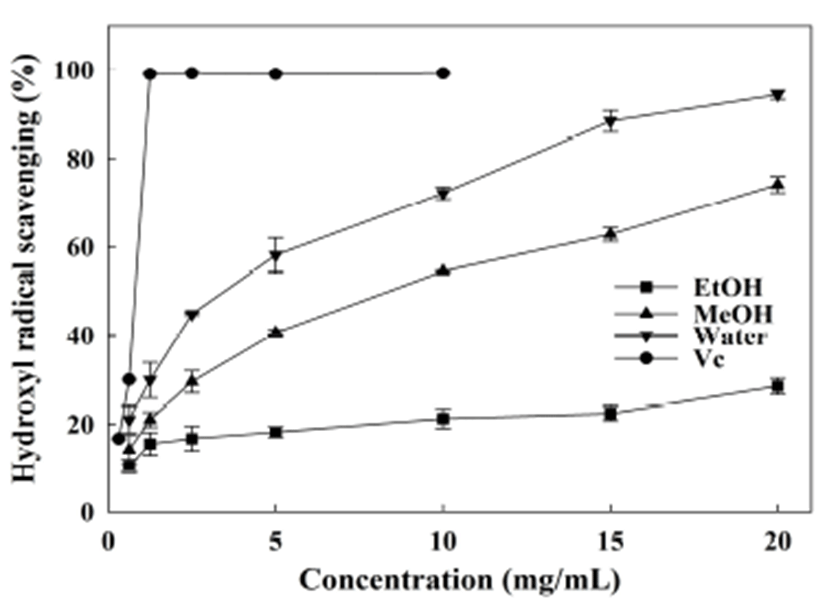
As the most reactive radical, the hydroxyl radical is believed to be deeply associated with DNA damage and lipid peroxidation. Fig. 2 illustrates that the hydroxyl radical was significantly scavenged by the water and methanol extracts in a dose-dependent manner. The scavenging ability ranged from 44.8% to 94.3%, and 29.5% to 74.1% for the water and methanol extracts respectively, for the extract concentrations from 2.5 to 20 mg/mL. However, ethanol extract did not exhibit remarkable activity at the same concentration range (16.7% to 28.4%). VC was used as positive control, due to its dominant antioxidant activity. The IC50 values of VC, water extract, and methanol extract were 0.72, 2.99, and 7.23 mg/mL, respectively (Table 2). As evaluated in a related study, water and 80% ethanol extracts from the A. hookeri root caused a significant reduction of hydroxyl radicals, and had IC50 values of 5.09 and 6.76 mg/mL, respectively (25). Thus, water extract obtained in current study was proven to be sufficient scavenger against hydroxyl radical. Previous study has demonstrated that alliin, allyl cysteine, and allyl disulfide can scavenge hydroxyl radicals, while allicin is not an effective scavenger (41). However, the scavenging activities of allicin, dimethyl thiosulfinate, and dipropenyl thiosulfinate have been reported by Xiao and Parkin (35). Thus, it can be deduced that the thiosulfinates in ethanol extract not contribute to the hydroxyl radical scavenging. In addition, the antioxidant capacity of leek was indicated directly correlated with the content of ascorbate more than total phenolics (42). Thus, the weak scavenging capacity of ethanol extract can be attributed to the deficiency of other effective compounds other than phenolic and thiosulfinate constituents.
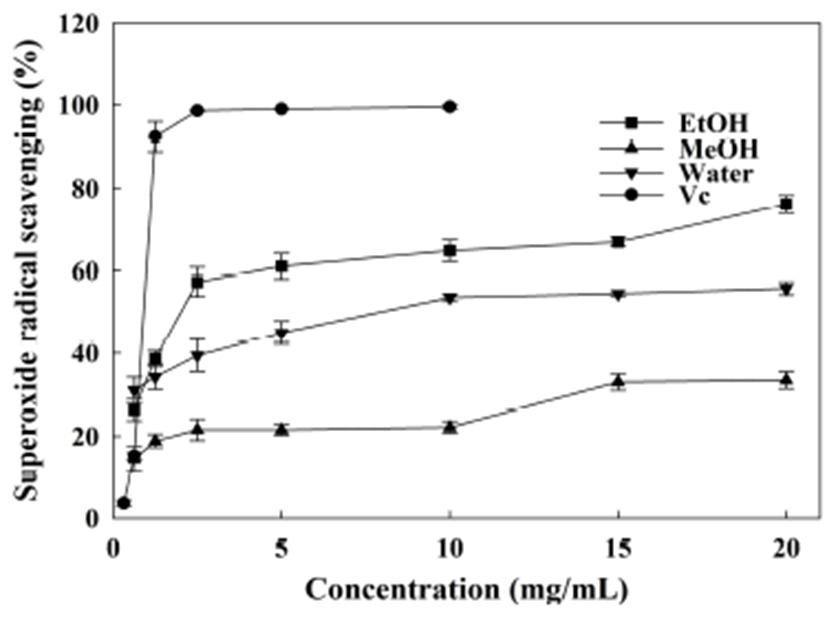
Superoxide radical is a highly toxic free radical that can be generated through various biological reactions. It is well known that superoxide radicals are the precursor of hydroxyl radicals and signet oxygen. Therefore, the superoxide radical scavenging assay plays an important role in the estimation of antioxidant capacity. Fig. 3 indicated that the ethanol and water extracts from A. hookeri root have strong abilities in the reduction of superoxide radical formation, while methanol extract showed very weak activity, comparatively. The reduced percentage of superoxide radicals caused by the ethanol extract was 54~76% at the dose level of 2.5~20 mg/mL (IC50 was 3.19 mg/mL). With respect to water extract, the reduced percentage of superoxide radicals was less (p<0.05) than that caused by ethanol extract (IC50 was 9.31 mg/mL). As compared with previous reports, similar effects were produced by the extracts obtained from different parts of mulberry (17), while more efficient effects were produced by diversified garlic extracts (43).
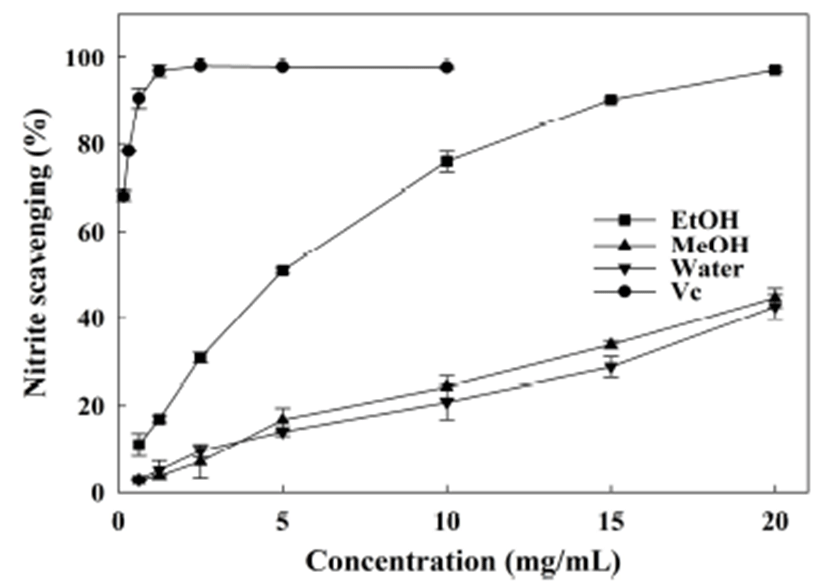
Nitrite is derived from nitrate, and can react with amine and amides in vivo to form N-nitroso compounds which may have carcinogenic properties. Furthermore, the overproduction of nitrite has been demonstrated to be involved in the oxidation of hemoglobin (22). Thus, the nitrite scavenging assay has been widely conducted to evaluate the antioxidant activity of natural components recently. It can be determined that the nitrite concentration decreases significantly with the increase of sample concentration (Fig. 4). The scavenging effect of ethanol extract was proven to be the highest among the extracts. The IC50 was 4.27 mg/mL for ethanol extract (Table 2), which was much lower than that of the water and the methanol extracts (32.14 and 26.48 mg/mL, respectively). The water extract of onion peels prepared under various conditions (44) scavenged nitrite by 21.71~33.97% at 10 mg/mL, slightly higher than that of water and methanol extracts from A. hookeri, while much lower than the ethanol extract in present study. Water and ethanol extracts from Gardenia jasminoides Ellis fruit (23) were indicated significantly scavenge nitrite (IC50 values were 1.09 and 1.58 mg/mL, respectively), implying the mediate nitrite scavenging activity of ethanol extract from A. hookeri root. In this assay, it can be found that phenolic, flavonoid, and thiosulfinate compounds may largely contribute to nitrite scavenging for the effect decreased in the order: ethanol extract>methanol extract>water extract.
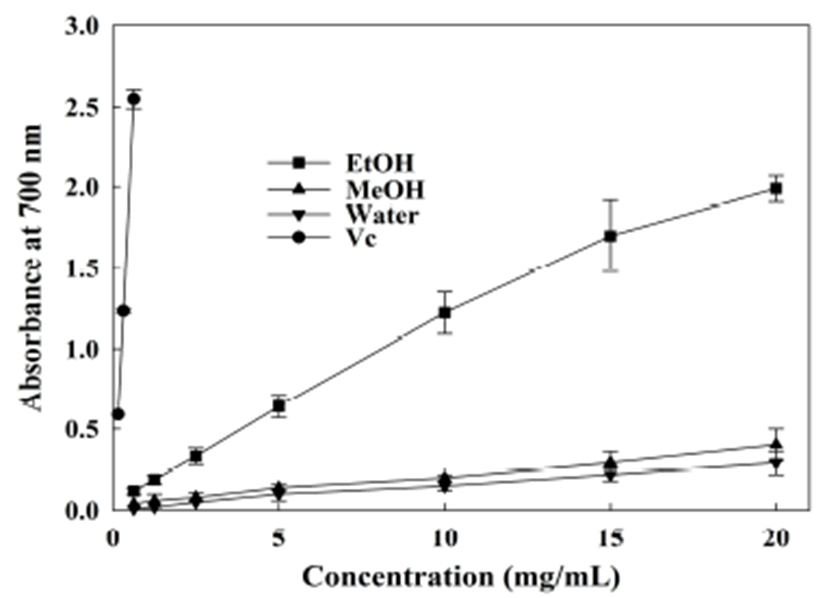
The reducing power assay conducted here was dependent on the reduction of Fe3+(CN−)6 to Fe2+(CN−)6 by the antioxidants. Perl’s Prussian blue, with a maximal absorption at 700 nm, will form subsequently with the addition of FeCl3. The increasing absorbance of the mixture in the presence of an antioxidant indicates more potent reducing power. In this study, the reducing power of extracts from the A. hookeri root was reported for the first time. Results indicated that the ethanol extract has the strongest reducing power (Fig. 5). At a concentration range of 2.5~20 mg/mL, ethanol extract contributed to a high absorbance of 0.33~1.99, much higher than that yielded by the methanol and water extracts (0.08~0.4 and 0.05~0.29, respectively). The IC50 for ethanol extract was 3.22 mg/mL, while for the other two extracts, it was much more than 20 mg/mL. As described previously, the methanol extract obtained from A. ampeloprasum exhibited efficient reducing power with IC50 value of 0.70 mg/mL (28), while the extracts from rosy garlic (A. roseum var. odoratissimum) did not reveal reducing power (45). Thus, A. hookeri extracts possess certain reducing power among the natural materials.
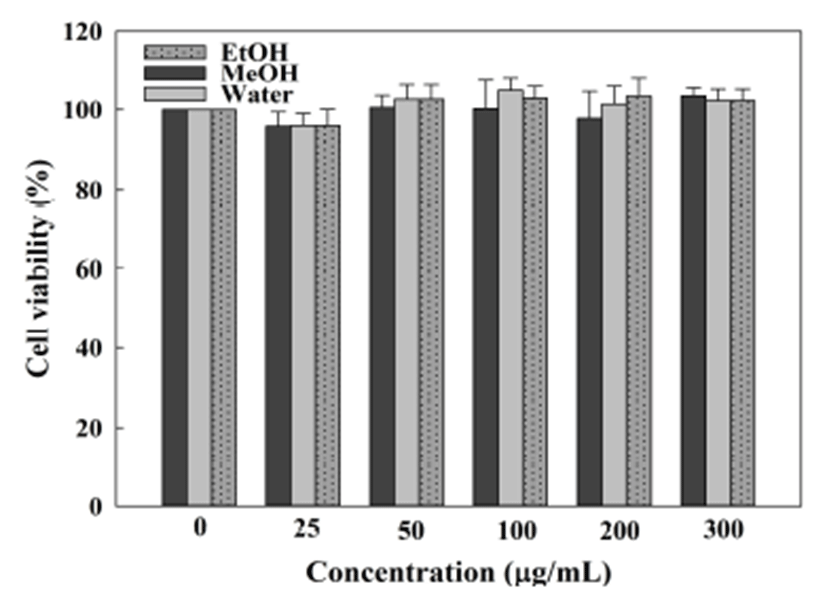
Cell viability measurement is based on the reaction between MTT reagent and the oxidative enzyme in viable cells. In this study, cells were treated with a sample of 0-300 μg/mL. After treatment with the extracts for 24 hr, the viability of the RAW 264.7 macrophages showed slight fluctuation around 100% and no significant difference was observed (Fig. 6). Therefore, it can be concluded that the tested samples have no cytotoxic effects on RAW 264.7 macrophages at the dose levels examined.
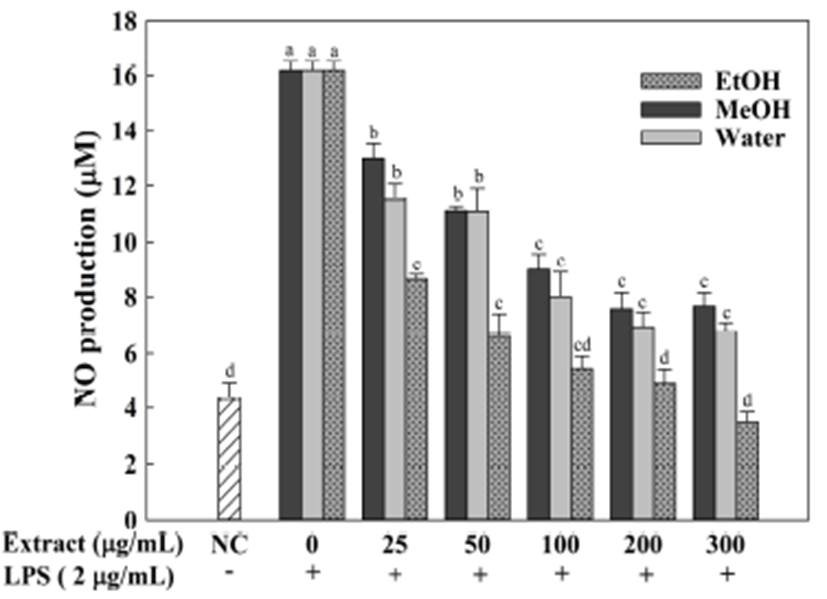
As shown in Fig. 7, A. hookeri root extracts exerted remarkably dose-dependent inhibitory activity on NO production. Among the samples, ethanol extract exhibited the most efficient activity on the suppression of NO production (IC50 was 29.13 μg/mL). In contrast to ethanol extract, considerably less is known about the anti-inflammatory effect of the water and methanol extracts (IC50 were 134 and 200 μg/mL, respectively). The anti-inflammation activity of A. hookeri root has been evaluated in the past and a potent effect has been demonstrated. A. hookeri extract prepared with 70% ethanol was demonstrated is able to inhibit the NO production and a strong effect was observed at 250 μg/mL (13). Kim et al. (14) estimated the anti-inflammatory property of 70% methanol extract, and the results revealed that the extract strongly suppressed the NO production. These reports also demonstrated that ethanol and methanol extracts from A. hookeri root decreased the cytokines (IL-6, IL-1β and TNF-α) production and inhibited the HO-1 and NF-κB activity.
According to previous studies, the abundant contents of phenolic, flavonoid, and thiosulfinate components may explain the excellent inhibitory activity of ethanol extract on NO production (10,32,36). Shin et al. (46) indicated that the reduced anti-inflammatory effects of garlic extracts were due to the reduction of allicin, implying the potent anti-inflammatory effects of allicin, even thiosulfinates. Kratchanov et al. (47) indicated that the polysaccharides obtained from leek have potent immunostimulating activities. Thus, other compounds more than phenolics and thiosulfinates mainly contribute to the anti-inflammatory activity of water extract. For methanol extract, the low anti-inflammatory effect may directly due to the low contents of phenolics and thiosulfinates.
In conclusion, this study revealed that extracts from A. hookeri root possessed remarkable antioxidant and anti-inflammatory properties. The activities of the extracts were found in descending order: ethanol extract>water extract>methanol extract. The polyphenolic, flavonoid, and thiosulfinate components may act as antioxidants and anti-inflammatory reagents, and contribute to the effects at different levels. Our findings provided evidence for the possible use of the A. hookeri root in medicine and functional foods.
요 약
본 연구에서는 삼채 뿌리를 물, 에탄올, 메탄올로 추출했 다. 추출방법에 따라 추출물의 항산화 및 항염증 효과는 다르게 확인됐다. 그 중에는 에탄올 추출물은 가장 좋은 효과를 확인하였으며 강한 환원력, DPPH 라디칼, superoxide 라디칼 및 아질산염 소거능이 나타났다 (IC50값 은 각각 3.22, 4.26, 0.95, 3.22 mg/mL). 물 추출물과 메탄올 추출물은 좀 약한 효과를 나타났는데 좋은 hydroxyl 라디칼 과 superoxide 라디칼 소거능을 볼 수 있다. RAW 264.7 세포를 통해서 측정한 결과에 따라 모두 추출물은 유익적인 염증 개성 효과를 나타냈다. 에탄올 추출물은 가장 높은 NO 생성 저해 활성을 나타내고 그 다음은 물 추출물과 메탄올 추출물을 순으로 나타냈다. Total phenolic, flavonoid 하고 thiosulfinate의 함량은 다 에탄올 추출물은 제일 높은 농도를 가지고 있다 (각각 24.96 mg GAE/g extract, 4.27 mg RE/g extract, 14.2 μM/g extract). 연구결과에 따라 삼채 뿌리는 높은 total phenolic, total flavonoid하고 total thiosulfinate 함량을 함유하고 좋은 항산화 및 항염증 효과 를 볼 수 있다.