Introduction
The incidence of diabetes is as high as 10% in Korea and patients with type 2 diabetes account for 90% of diabetic population (1). Despite much headway in diabetes research, the management of diabetes is still a formidable challenge in clinical practice. Previous studies have proved that decreasing the postprandial hyperglycemia is a feasible strategy to treat the early stage of type 2 diabetes. Inhibition of key enzyme for carbohydrate metabolizing such as α -amylase and α-glucosidase can effectively delay the absorption of glucose, and consequently blunt the postprandial plasma glucose rise (2,3). Natural plant products or extracts such as tea and raspberry, which exhibited significant inhibitory activities on α-gluosidase, are recommended to replace the synthetic drugs for type 2 diabetes patients to improve their postprandial hyperglycemia (4-6).
Oxidative stress occurs in the cell and plasma when the generation of reactive oxygen species (ROS) including superoxide radicals, hydroxyl radicals and hydrogen peroxides upsets the redox balance and leads to cell dysregulation (7). Accumulated evidence has also supported that increased oxidative stress is associated with diabetes and its complications (7,8). Antioxidant supplements are reported to be meaningful and essential to reduce the generation of ROS and strengthen antioxidant defense system (9). Natural antioxidants with very less side effects have received considerable attention compared to the synthetic antioxidant components.
The commercial vegetable extract used in our study, Ya Chae Soo, is very popular in Korea. The raw material for producing Ya Chae Soo included five edible vegetables, namely white radish (Raphanus sativus L.), carrot (Daucus carota L.), burdock (Arctium lappa), shiitake (Lentinus edodes), and radish tops. Radish, carrot, burdock, shiitake, and radish tops have been extensively studied for their nutritional characteristics, compound composition and biological activities including antioxidant, anticancer, antimicrobial, antipyresis activities and detoxifying effects (10-13). Seaweed, one of the most important marine resources, has been utilized traditionally as food supplement in Korea and other Asian countries. In recent years seaweeds have been proved to contain various components, which are effective antioxidants (14) and also exhibit particular biological activities. For example, Alaria, Palmaria, and Ascophyllum extract significantly inhibited proliferation of cancer cell (15) and extract from Turbinaria ornate had strong anti-inflammatory activity (16). More recently, seaweed extract with large amount of phenolic compounds have been suggested to suppress the postprandial hyperglycemia through inhibition of starch digestive enzymes (17). However, the effect of seaweed on nutritional quality and physiological activities including antioxidant and antihyperglycemia potential of commercial vegetable extract like Ya Chae Soo has not been investigated.
Therefore, in the present research, the influence of addition of Hizikia fusiforme, Capsosiphon fulvescens, Undaria pinnatifida sporophyll extracts at different addition levels (5, 10 and 20%) on antioxidant and inhibitory potential against key enzymes linked to hyperglycemia of commercial vegetable extract were systematically investigated. Additionally, the nutritional qualities of vegetable extracts added with seaweed were also studied.
Materials and methods
Ferrous sulfate, sodium salicylate, 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydrogen peroxide, ferric chloride, α -glucosidase from saccharomyces cerevisiae (EC 3.2.1.20), soluble starch, butylated hydroxyanisole (BHA), potassium sodium tartrate tetrahydrate, gallic acid, p-nitrophenyl-α -D-glucopyranoside, potassium ferricyanide, porcine pancreatic α-amylase, type VI-B (EC 3.2.1.1), trichloroacetic acid, Folin & Ciocalteu,s phenol reagent, 3,5-dinitrosalicylic acid, aluminum nitrate nonahydrate, sodium carbonate, sodium nitrite were obtained from Sigma Chemical Co., (St. Louis, MO, USA). All other chemicals and solvents used were of standard analytical grade.
The commercial vegetable extract was kindly provided by Hyundai Agricultural Association (Yuwol-ri, Haeje-myeon, Muan-gun, Jeollanam-do, Korea). The Hizikia fusiforme (HF), Capsosiphon fulvescens (CF), and Undaria pinnatifida sporophylls (UPS) were collected from a clean region of Wando, Korea. Fresh seaweeds were washed with tap water and dried at 50°C. These samples were then ground and sieved to obtain fine powder. Powdered seaweed materials were mixed with distilled water (1:25, w/v) and extracted at 121°C for 3 h. The filtrate was collected in bottles after vacuum filtration (Whatman No.1 filter paper). The acquired seaweed extract was formulated with vegetable extract at three different levels including 5, 10, and 20% (Table 1).
| Ingredients | S01) | S5 | S10 | S20 | S100 |
|---|---|---|---|---|---|
| seaweed extract | 0 | 5 | 10 | 20 | 100 |
| vegetable extract | 100 | 95 | 90 | 80 | 0 |
| total | 100 | 100 | 100 | 100 | 100 |
The moisture, crude fat, protein and ash contents of samples were determined in accordance with the standard methods of the AOAC (18). Carbohydrate contents were calculated as the difference of 100-(crude ash+crude protein+crude fat+moisture). The concentrations of the mineral (calcium, sodium, potassium, magnesium, iron) were determined by atomic absorption spectrophotometer (spectraAA 220 Fs, Varian Co., Mulgrave, Victoria, Australia).
The TPC were determined according to the standard Folin-Ciocalteau method (19). 0.1 mL of seaweed extract solution were placed in a tube, to which 7.9 mL of deionized water and 0.5 mL of Folin-Ciocalteau solution were added. 15 min later, 1.50 mL of 1.85 M sodium carbonate solution was added and the tube was kept in darkness for 2 h. Absorbance were measured at 765 nm and the TPC were obtained from standard curve of gallic acid (y=0.9977x, R2=0.9915).
The DPPH radical scavenging activity was determined by the method of Yang et al. (20). Four milliliters of reaction mixture contained 2.0 mL of 0.1 mM DPPH solution and 2 mL sample solution at various concentrations. The mixtures were shaken vigorously and placed in darkness. Absorbance was measured at 517 nm after 30 min and the inhibition ability was obtained from the formula (1):
Hydroxyl radical scavenging activity was measured according to the method of Smirnoff and Cumbes (21). 0.3 mL of 20 mM sodium salicylate solution was mixed with 1.0 mL of 1.5 mM ferrous sulfate solution and 1.0 mL of seaweed extract solutions in a tube, to which 0.7 mL of 6 mM H2O2 were added to initiate reaction. The reaction mixture was incubated at 37°C for 1 h. The absorbance was read at 562 nm and the inhibition ability was obtained using formula (1).
The reducing power was determined by the previously described method (22). Seaweed extract solution (0.5 mL) was placed in a tube, to which 1.25 mL of phosphate buffer solution (0.2 M, pH 6.6), as well as 1.25 mL of 1% potassium ferricynide solution were added. After incubation at 50°C for 20 min, 1.25 mL of trichloroacetic acid solution were added to the tube. 1.25 mL of supernatant obtained by centrifugation at 3,000 rpm for 10 min was diluted with 1.25 mL of deionized water. Then, 0.25 mL of 0.1% ferric chloride solution was added to complete the assay. The absorbance was determined at 700 nm and represented the reducing power.
The inhibitory potential on α-amylase was measured according to the method of Ranilla et al. (4). 0.5 mL of seaweed extract solution was placed in a tube, to which 0.5 mL of 0.5 mg/mL α-amylase solution was added. After preincubation at 25°C for 10 min, 0.5 mL of 1% soluble starch solution were added to the tube. The tubes were then kept at 25°C for 10 min. 1.0 mL of dinitrosalicylic acid color reagents was added to each tube to terminate the reaction. The tubes were allowed to keep at 100°C for 5 min. Finally the reaction mixture in the tube was diluted with 10 mL of deionized water as it cooled to room temperature. The absorbance was determined at 540 nm and the inhibition ability was obtained using formula (1).
Inhibition effect on α-glucosidase was evaluated by the previously described method (17) with some modification. 0.5 mL of seaweed extract solution prepared by phosphate buffer (100 mM, pH 6.9) was mixed with 0.5 mL of 5 mM p-nitrophenyl-α-D-glucopyranoside (in phosphate buffer, pH 6.9). After incubation at 37°C for 5 min, 1 mL of phosphate buffer solution containing α-glucosidase (0.1 U/mL) was added into test tube at time interval. Finally the absorbance was measured at 405 nm after incubation at 37°C for 10 min and the inhibition ability was obtained using formula (1).
Results and discussion
As shown in Table 2, there were no significant difference between vegetable extract supplemented with seaweeds and pure vegetable extract for moisture, crude protein, crude fat, crude ash, and carbohydrate content. In contrast, mineral content was meaningfully increased in a seaweed addition level dependent manner. Potassium (K) was the predominant element present in the pure vegetable extract, followed by sodium (Na), calcium (Ca), and magnesium (Mg). The iron (Fe) content was scarce in the pure vegetable extract (Table 3). Expect for K content of Capsosiphon fulvescens extract, the mineral content of Hizikia fusiforme, Capsosiphon fulvescens, and Undaria pinnatifida sporophyll extracts was much higher than that of pure vegetable extract. When Hizikia fusiforme extract was added into vegetable extract at 5% level, the content of Ca, Na, K, Mg, and Fe were significantly (p 0.05) increased by 13.91, 10.34, 10.79, 35.33, and 31.26%, respectively. And when Undaria pinnatifida sporophyll extract was added into vegetable extract at 5% level, apart from Ca, the contents of Na, K, Mg, and Fe were significantly (p˂0.05) increased by 27.28, 8.21, 27.67, and 41.91%, respectively. In the case of vegetable extract added with Capsosiphon fulvescens at 5% level, the concentration of Mg and Fe were significantly (p˂0.05) increased by 30.27 and 28.82%. The increase in mineral content of vegetable extract can be attributed to the high mineral content of Hizikia fusiforme, Undaria pinnatifida sporophyll, and Capsosiphon fulvescens extracts.
In the present study, the total phenolic content of vegetable extract which was 15.40 mg GAE/100 mL, was significantly (p˂0.05) increased to 20.35 mg GAE/100 mL when supplemented by Hizikia fusiforme extract at 10% level (Fig. 1). However, significant increase of total phenolic content was not found in vegetable extract added with Undaria pinnatifida sporophyll and Capsosiphon fulvescens extracts. All of these results can be attributed to the higher total phenolic content of Hizikia fusiforme (40.29 mg GAE/100 mL) and relatively lower total phenolic contents of Capsosiphon fulvescens and Undaria pinnatifida sporophyll (12.03-17.06 mg GAE/100 mL). Son et al. (23) also reported that the total phenolic content of carrot juice was influenced by the addition level (1, 3, and 5%) of beet extract. The total phenolic content (5.49 mg GAE/100 mL) of carrot juice was increased to 11.42 mg GAE/100 mL with the addition of beet extract at 5% level.
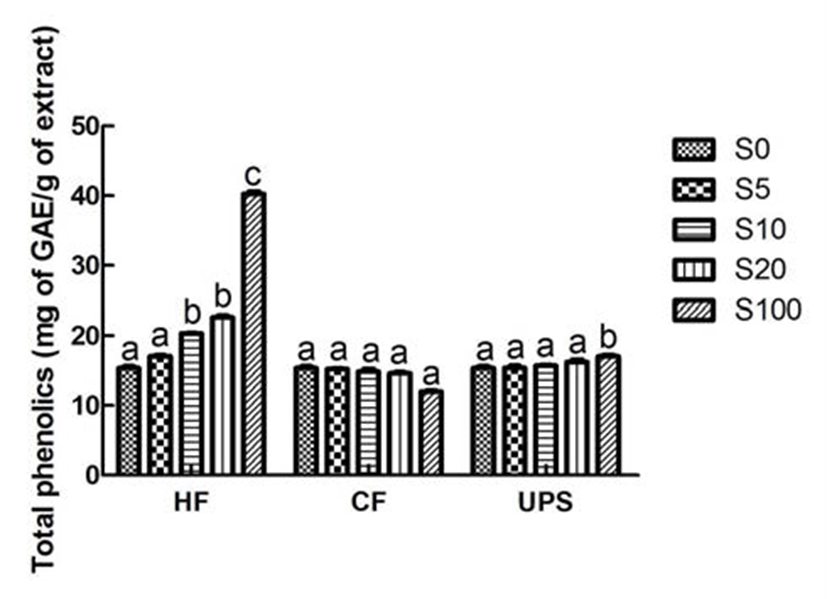
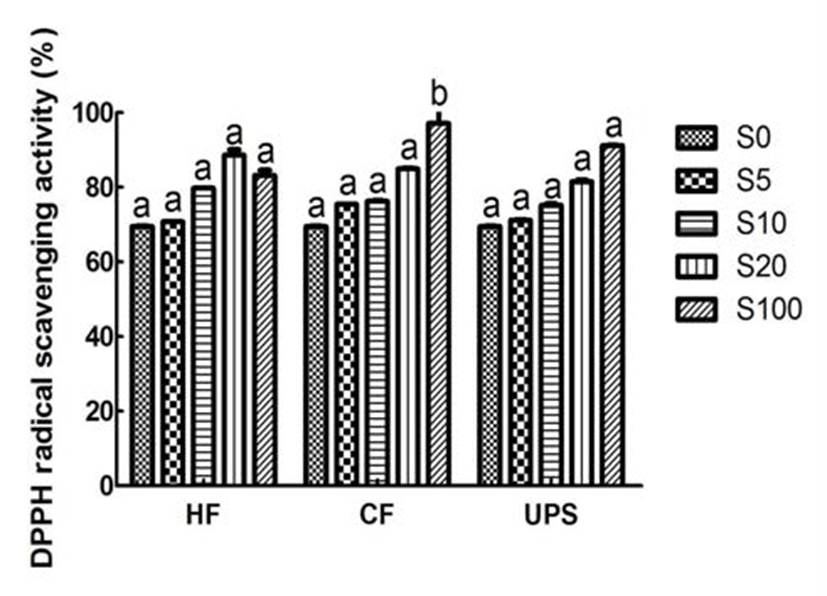
As can be seen from Fig. 2, the vegetable extract exhibited a relatively higher DPPH scavenging ability (69.52%). This higher DPPH radical scavenging activity may be due to the higher total phenolic compound content of vegetable extract. The vegetable extract added with seaweed scavenged DPPH radical in an addition level dependent manner. With the addition of seaweed extracts, the DPPH scavenging activities were gradually increased. In particular, when Hizikia fusiforme, Capsosiphon fulvescens, and Undaria pinnatifida sporophyll were added into vegetable extract at 20% level, the DPPH radical scavenging activities were significantly (p˂ 0.05) increased by 27.47, 22.25, and 17.27%, respectively. Previous study (24) in this area had also reported the similar addition effect (7, 14, 21, and 28%) of maca (Lepidium meyenii) on DPPH radical scavenging activity of syrup.
It can be observed that the hydroxyl radical scavenging activity of vegetable extract was good (53.64%). However, in contrast to the DPPH radical scavenging performance, when adding Hizikia fusiforme into vegetable extract, the scavenging activities on hydroxyl radical were not found to be changed at each addition level (Fig. 3). The hydroxyl radical scavenging activity of vegetable extract was increased, though not significantly, with the addition of Capsosiphon fulvescens, and Undaria pinnatifida sporophyll at 20% addition level. In addition, Chung et al. (24) also reported that the addition of maca into syrup did not increase the hydroxyl radical scavenging activity.
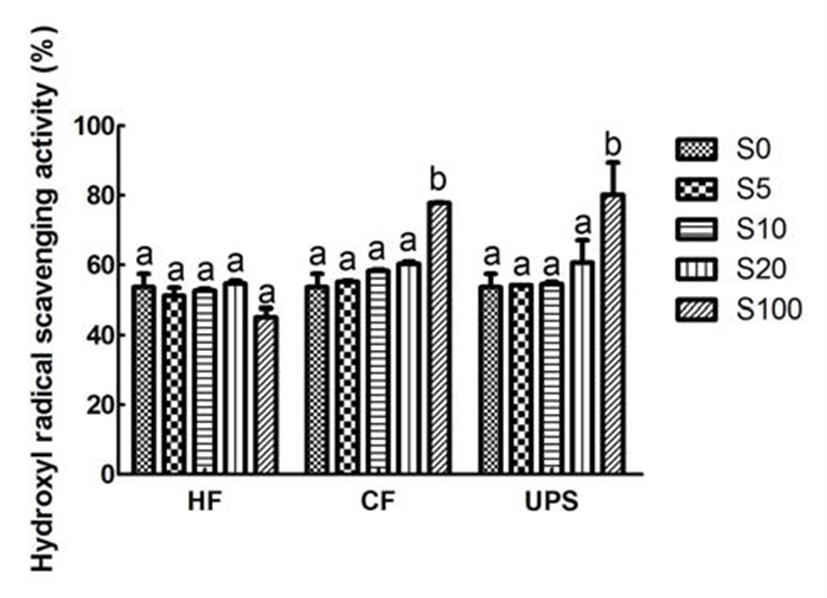
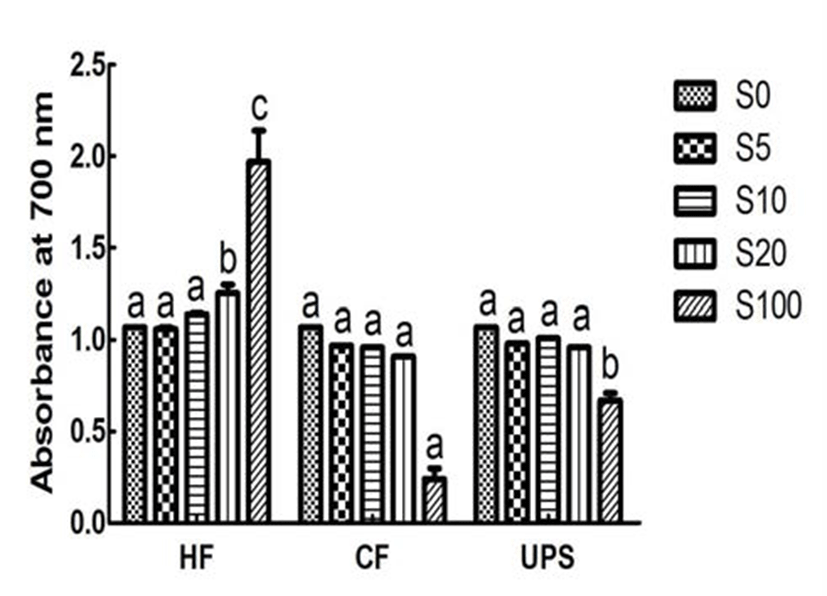
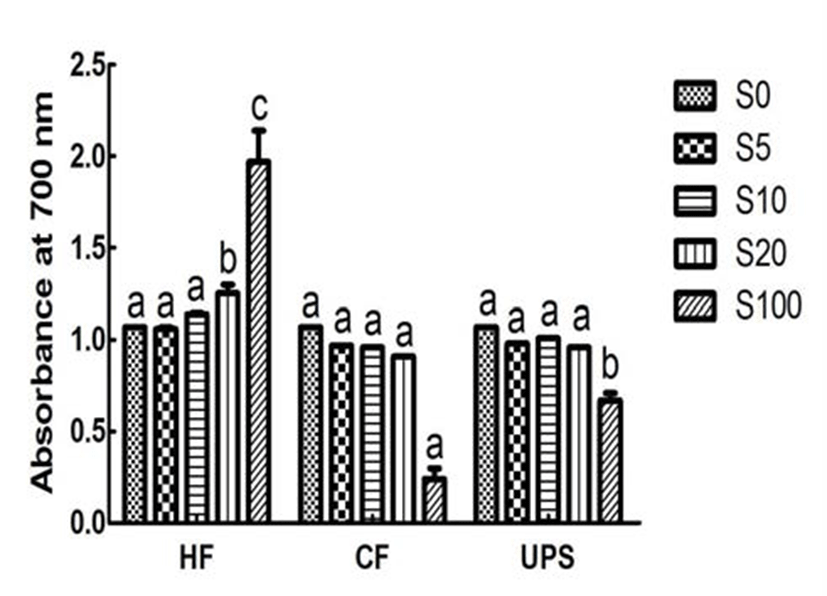
Fig. 4 showed the reducing power, an important indicator of antioxidant ability, for vegetable extract added with seaweeds and pure vegetable extract. The reducing power of vegetable extract, in term of absorbance at 700 nm, was 1.07. The reducing power was significantly (p 0.05) increased by 16.82 % when adding 20% level of Hizikia fusiforme into the vegetable extract. In contrast, addition of Capsosiphon fulvescens and Undaria pinnatifida sporophyll extracts did not significantly change the reducing power (0.91~1.01). This can be partly explained by the low or medium reducing power (0.24~0.67) of Capsosiphon fulvescens and Undaria pinnatifida sporophyll extracts and higher reducing power of Hizikia fusiforme extract (1.97).
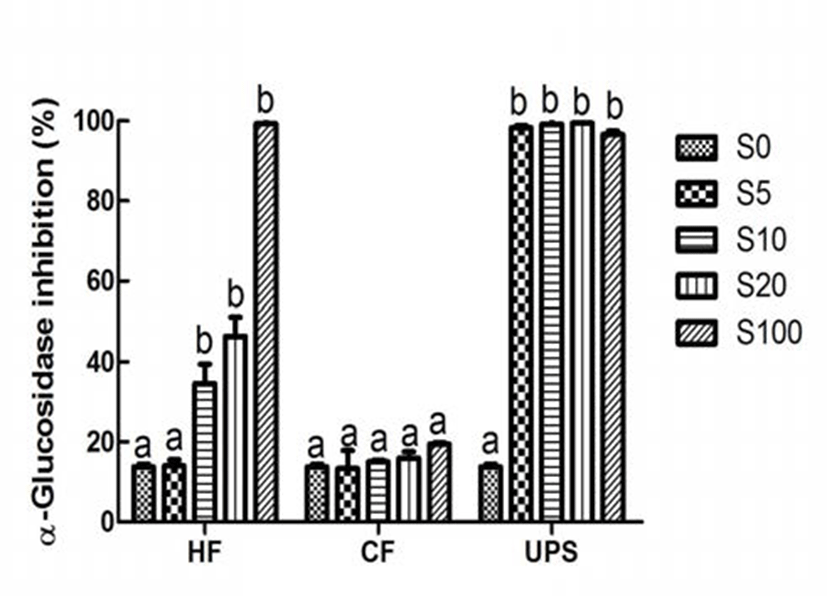
α-Amylase and α-glucosidase inhibitors, especially these, which exhibited stronger inhibition effect on α-glucosidase and weaker inhibition effect on α-amylase, can make a big difference in the prevention and improvement of hyperglycemia (25). Different from the performance of antioxidant, which were relatively strong or medium, the inhibition activity of pure vegetable extract on type 2 diabetes related enzymes, namely α-amylase and α-glucosidase, was very weak (10.82 and 13.77%). This is in contrast to the results of earlier studies (4,6), which have revealed that plant extracts rich in total phenolic content had weaker or no inhibition effect on α-amylase and stronger inhibition effect on α-glucosidase. This may be attributed to the differences of experimental methods and phenilc compounds presented in extracts. However, with the addition of Hizikia fusiforme, Capsosiphon fulvescens, and Undaria pinnatifida sporophyll extract at 20% level, the α-amylase inhibitory activity of vegetable extract was significantly (p˂0.05) increased by 97.87, 103.51, and 211.37%, respectively. In the case of α -glucosidase inhibition activity, addition of Capsosiphon fulvescens extract did not change the α-glucosidase inhibition activity of vegetable extract. When Hizikia fusiforme extract was added into vegetable extract at 10% level, the α -glucosidase inhibition activity was significantly increased (p 0.05) by 151.42%. It is worth noting that the α-glucosidase was almost completely inhibited when Undaria pinnatifida sporophyll extract was added into the vegetable extract at 5% level. In addition, vegetable extract added with Undaria pinnatifida sporophyll had a stronger α-glucosidase inhibition activity and a weaker α-amylase inhibition activity, indicating that Undaria pinnatifida sporophyll, which are underutilized and usually damped as a fishery waste, can be used as a functional food ingredient of commercial vegetable extract to improve the type 2 diabetes related postprandial hyperglycemia.
In conclusion, our results revealed that the commercial vegetable extract had relatively low mineral contents and exhibited medium or weak antioxidant and antihyperglycemia activities. Addition of seaweed extracts such as Undaria pinnatifida sporophyll, Hizikia fusiforme and Capsosiphon fulvescens extracts, can significantly increase the mineral contents and improve antioxidant activity and inhibitory potential against type 2 diabetes related enzymes. Thus, this study also indicated that utilization of these seaweed extracts in the development of novel vegetable beverage would be an important step toward promoting health.Fig 56
요 약
혼합야채추출물의 항산화와 α-amylase 및 α-glucosidase 억제활성 증진을 위해서 톳, 매생이, 미역귀의 첨가효과를 분석하였으며 해조류 첨가된 야채추출물의 일반성분과 미 네랄 함량 변화를 조사하였다. 3가지 해조류의 첨가량은 야채추출물의 일반성분에 유의적으로 영향을 미치지 않은 반면 톳과 미역귀 5% 첨가에 의해 칼슘, 나트륨, 칼륨, 마그 네슘, 철분 함량이 유의적으로 증가하였다. 톳 20% 첨가한 경우 총 폴리페놀 함량과 환원능력이 야채추출물보다 각각 47.08와 16.82%로 유의적으로 증가하였다. 반면에 3가지 해조류 첨가에 의한 hydroxyl radical 소거활성이 큰 변화가 없었다. 톳, 매생이, 미역귀 20% 첨가에 의해 DPPH radical 소거활성이 각각 27.47, 22.25, 17.27%로 유의적으로 증가 하였다. 해조류 첨가된 혼합 야채 추출물이 상대적으로 약 한 α-amylase 억제활성을 지니고 있는 동시에 강한 α -glucosidase 억제활성이 나타냈다. 특별히 미역귀 5% 첨가 한 경우 α-glucosidase을 98.26%로 유의적으로 억제하였다. 전반적으로 해조류 첨가를 통해 야채추출물의 미네랄 함량 이 증가하였고 항산화와 2형 당뇨병 관련 효소 억제활성이 개선됨을 알 수 있었다.