Introduction
Jeotgal, prepared by various seafood, such as shrimp, oysters, shellfish, fish eggs and also fish intestines, is a salted fermented food in Korea and many other countries. The manufacture method of Jeotgal varies depending on the main ingredients, regions, and personal preferences but the basic procedures are the same. Traditionally, the seafood is treated with 20% to 30% of salt as the main ingredients. The fermentation is allowed from several months to even several years, so that to form the favored taste that Jeotgal owns (1). Jeotgal is mainly used in the pickling kimchi as a condiment or as a side dish alone. Jeotgal is valued for its distinctive taste as well as its high nutritional content (2). In recent years, the health promoting potential of Jeotgal, include the antioxidant activity, angiotensin I converting enzyme inhibition potential and the probiotics, have been investigated and discussed (3-5). On the other hand, the manufacture procedures and the main ingredients of Jeotgal are also under development as more and more novel Jeotgal have been proposed (6-8).
Abalone, Haliotis Discus Hannai Ino, is an important aquatic economic species widely cultured in East Asia, Australia, America and many other regions. The production of abalone has increased greatly in the recent two decades with the introduction of farm aquaculture. It is estimated the world total output of abalone is more than 30,000 metric tons per year (9). Generally, the visceral part of abalone is not regarded as edible by the producers as well as customers. The viscera is treated as by-product and most of them is discarded directly or used as fish silage (10,11). However, the nutritional and healthy value of the visceral part of abalone should not be overlooked since more and more researchers have paid great attention to the bio-activities and health promoting ability of the abalone viscera. Sun L et al. (12) discussed the antioxidant activities of a sulphated polysaccharide obtained from the abalone viscera. Ping K and Qiukuan W (13) studied the potential application of the enzyme extract of abalone viscera. Several other articles mentioned the antioxidant potential of polysaccharide extracted from abalone viscera (14,15). The application of abalone viscera as the food ingredient has also been researched (16). Our previous study has discussed the anti-skin-aging activities of extract from abalone viscera (17). The results of all the researches indicate that abalone viscera possess very high nutritional and functional value, suggesting its more widely application possibility.
In this study, we prepared abalone viscera Jeotgal and determined the optimum manufacture conditions. We also examined the physicochemical properties of the proposed Jeotgal. At last, the response of the customers to this abalone viscera Jeotgal was investigated. Since this is the first research that discussing the abalone viscera Jeotgal, it will provide the basis for the future related researches.
Materials and methods
Abalone was obtained from the aquatic market of Wando-gun in February 2012. All the abalone was shucked and eviscerated. After that, the abalone viscera was gathered, homogenized (HMF-3260S, Hanil Electric, Seoul, Korea) for 30 min under room temperature and stored at -20℃ in refrigeratory until use. After thawing, 1 kg of abalone viscera and 130 g, 150 g, 180 g, 200 g and 250 g of solar salt (Taepyung Salt Co., Sinan-gun, Korea) was mixed respectively in a plastic vat. The plastic vat was sealed and placed for fermentation and maturation at the temperature of 10℃ for 60 days.
Moisture content, crude protein content, crude lipid, ash and carbohydrate content were investigated according to methods described in the AOAC (1990). Conversion factor for the examination of protein content was 6.25.
The Jeotgal was pretreated before the pH was examined. First, the sample was homogenized with 4 times (v/w) of distilled water in a mixer (HMF-3260S, Hanil Electric) for 5 min. The sample was then transferred to a centrifuge tube for centrifugation at 300×g for 20 min (1736R, Hanil Electric). The supernatant was filtered using a filter paper and the filtrate was measured for the pH variation during the Jeotgal fermentation period using a pH meter (730P, Isotec. Inc., Miamisburg, OH, USA).
The VBN was determined by the Conway's method in a conway unit with some modifications (18). The sample (10 g) was mixed with equal amount of distilled water and twice amount of 10% trichloroacetic acid solution and grinded in a mortar for 5 min. After grinding, the sample was filtered and the remnant was grinded and washed with 5% trichloroacetic acid solution. All the sample and solution was filtered and transferred to a 50 mL volumetric flask to which 5% trichloroacetic acid solution was used to fill. Pretreated sample (1 mL) was extracted and transferred to the outer room of the conway unit with 1 mL of 1% H3BO3 solution in the inner room. Saturated K2CO3 solution (1 mL) was mixed carefully with the sample solution in the outer room and the conway unit was sealed and incubated at 37℃ for 1 hr. The mixture in the outer room was neutralized with 0.02 N H2SO4 solution until the H3BO3 solution turned to pink color. VBN value was calculated in the following equation.
VBN (mg/g) = 0.28×(V1-V2)×5000/S
In the equation: V1 is the volume (mL) of 0.02 N H2SO4 solution in the experiment;
V2 is the volume (mL) of 0.02 N H2SO4 solution in the control experiment in which equivalent amount of distilled water was used in place of sample solution;
0.28 is the result of 14 (molecular weight of nitrogen) multiply with the concentration of H2SO4 solution, 0.02; S is the weight of the sample.
AN was estimated with the Formol method (18). The sample was grinded and mixed with 50 mL of distilled water. The filtrate (25 mL) of the sample was added with 25 mL of formaline solution (30 mL) adjusted to pH 8.4 by 0.1 N NaOH and 30 mL of distilled water. The solution was titrated using 0.1 N NaOH to pH 8.4.
AN value was calculated in the following equation.
AN (mg/g) = (A-B)×280/S
In the equation: A is the volume (mL) of 0.1 N NaOH solution used in the titration in the experiment;
B is the volume (mL) of 0.1 N NaOH solution used in the titration in the control experiment in which equivalent amount of distilled water was used in place of formaline solution;
solution; 280 is the result of dilution factor (50 mL/25 mL) multiply with 14 (molecular weight of nitrogen) and divide the concentration of NaOH 0.1 N.
S is the weight of the sample.
The protease activity of the abalone viscera Jeotgal was determined by the protocol of Anson (19). The crude enzyme extract was prepared with grinded sample (6 g) extracted in 100 mL distilled water for 1 hr before filtration. The crude enzyme extract (0.5 mL) was mixed with 2 mL of 0.6% casein solution (pH 7.2) used as the substrate and the react was carried out at 30℃ for 10 min. The reaction was stopped by adding 2.5 mL of 0.4 M trichloroacetic acid (TCA). After filtering, 1 mL of the filtrate was mixed with 5 mL of 0.4 M Na2CO3 and 1 mL of folin solution and the reaction was proceed for 20 min. The absorption was measured at 660 nm for amino acid liberation. One unit of protease activity was defined as the amount of enzyme to liberate 1 μM tyrosine under the experimental conditions.
The free amino acid was extracted with twice of 5% trichloroacetic acid solution for 24 hr. Once extracted, the solution was centrifuged at 10,000×g for 10 min. The supernatant was neutralized by three times of 0.05 M hydrochloric acid and then diluted with five time distilled water. All the samples were filtered with 0.45 μm membrane filter and the free amino acid composition was analyzed by the Hitachi L-8800 amino acid analyzer (Tokyo, Japan) according to the method described previously (20,21).
The sensory evaluation was undertook by a panel composed by 40 students in the food engineering department after they have received related education. Characteristics include color, taste, flavor and overall acceptability were evaluated and all the characteristics were scored from 1 (extremely unacceptable) to 5 (extremely acceptable) (22). The control groups were oyster Jeotgal, abalone meat Jeotgal and changran Jeotgal (aged and seasoned intestine of Alaska pollack) were all purchased from the same aquatic products market. The total score of each sample got represented its quality and likeness by the consumers.
Results and Discussions
The general composition of abalone viscera before the fermentation was measured. Moisture, protein, lipid, ash and carbohydrate content of abalone viscera (results listed in table 1) was influenced by several factors such as the abalone species, harvested regions, harvested time, feed, abalone age and other factors, as have been studied by several previous articles (23-26). Our research result confirmed with what have been published that moisture content ranged approximately 70%~80%, protein content ranged approximately 1.0%~5.0%, lipid content ranged approximately 0.6%~1.5%, ash content ranged approximately 2.0%~5.0% and carbohydrate content ranged approximately 16%~20%. The nutrition contained in the abalone viscera not only endow abalone viscera Jeotgal with the distinct flavor but also provides an appropriate media for the microorganisms involved in the following fermentation.
| Moisture | Protein | Lipid | Ash | Carbohydrate |
|---|---|---|---|---|
| 72.74±0.05 | 3.24±0.46 | 0.82±0.03 | 4.06±0.05 | 19.14±0.37 |
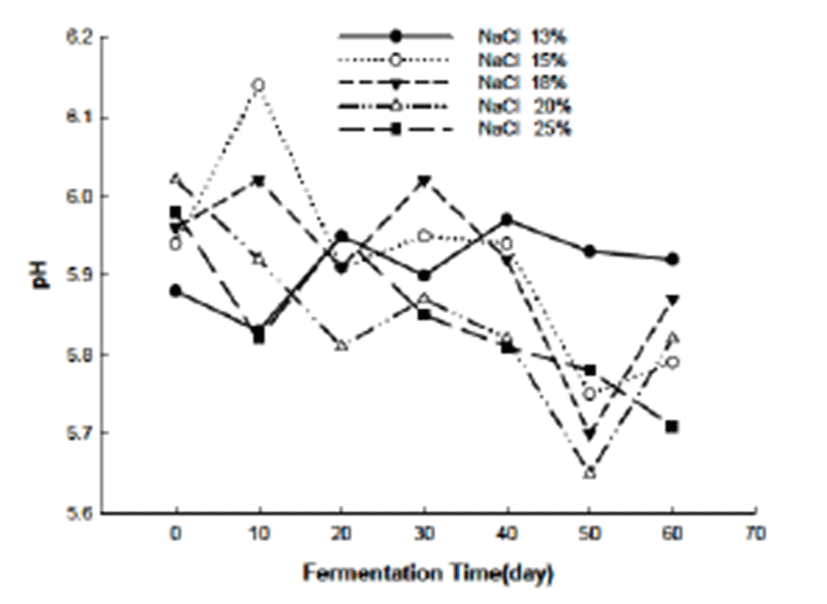
The pH variation during the fermentation was showed in the Fig. 1. As we can see in the figure, at the beginning of the fermentation (0 day) the pH scale of all the Jeotgal was 5.88~6.03 which fell into the normal pH scale for shellfish (5.5~6.5). During the fermentation the pH fluctuated from 5 to 6. The influence of the salinity to the pH of the Jeotgal was not believed in this study even though the Jeotgal added with 13% of salt presented higher (5.96) pH than the other Jeotgal added with more salt at the end of the fermentation. Generally, the pH variation is caused by the microorganism activity during fermentation. Hence, the pH variation is influenced by all the factors that have impact on the growth of the microorganism. In this study, the different amount of salt added to the abalone viscera may have affected the growth of the microorganism but the impact did not revealed in the pH variation.
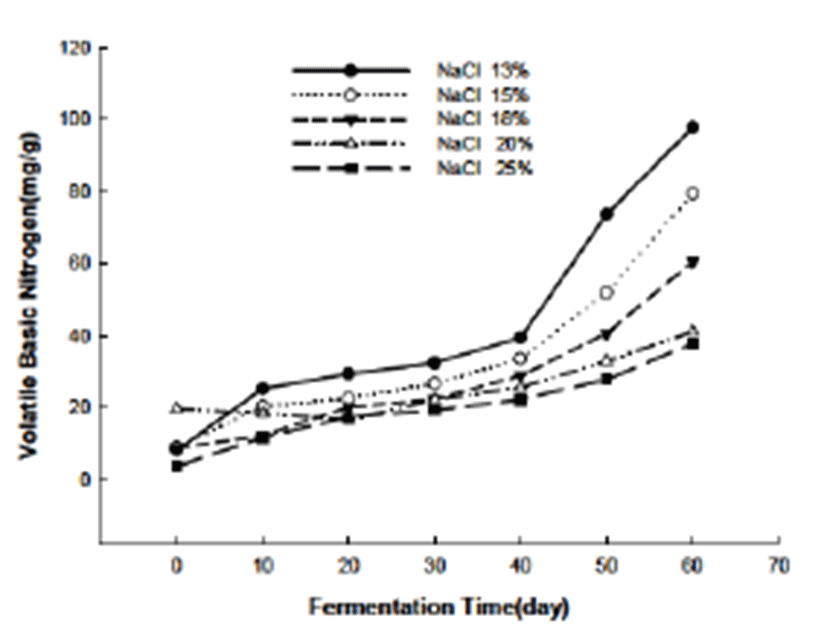
The influence of different salinity is more significant on the VBN change during the fermentation process as revealed in the Fig. 2. VBN is widely used as the indicator of fish and meat quality (27,28). While in this study, VBN is more a factor for fermentation than the freeness index since at the end of the fermentation the VBN of the Jeotgal exceeded the rejection value for most of the free fish (29). At the beginning of the fermentation, the VBN value ranged from 3.7 mg/g to 20.3 mg/g and as the fermentation proceeded the VBN value increase accordingly. At the end of the fermentation (60 days) the effect of the salinity on the VBN was more clearly. Low salt adding Jeotgal showed higher VBN value (96.7 mg/g) than other Jeotgal with higher salinity. The VBN value decreased in accordance with the increase of the salinity. Jeotgal added with 25% salt at the end of the fermentation presented (37.4 mg/g) less than a half of that of the Jeotgal added with 13% salt (96.7 mg/g). The difference of the VBN value among the different Jeotgal groups is attributed to the difference amount of salt added. Since the high salinity would inhibited the growth of the microorganism involved in the fermentation so that the release of the volatile basic nitrogen substances.
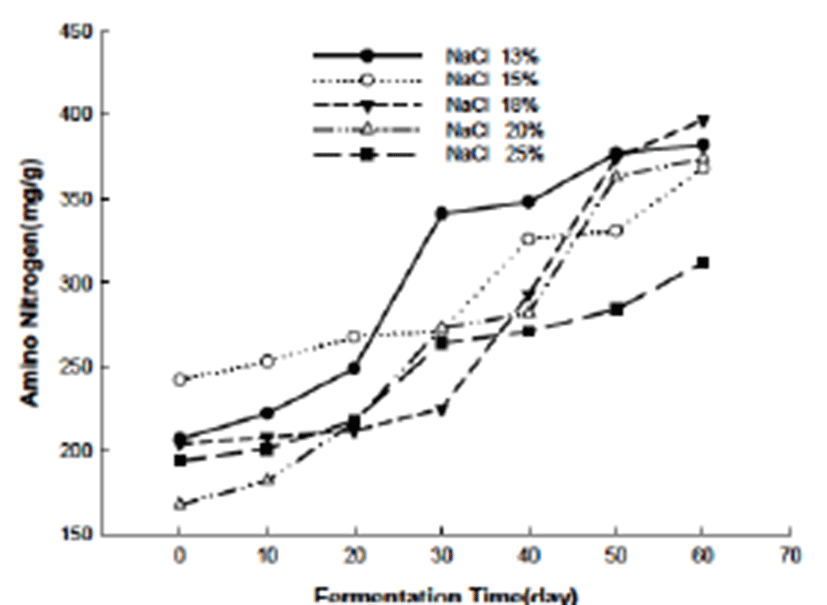
Amino nitrogen (AN) is related with the hydrolysis of the protein contained in the viscera. With the fermentation, protein was either decomposed by the autolysis or the effect of the microorganism happened during the fermentation (30, 31). Also AN is usually believed to confer the special flavor and aroma to Jeotgal. The AN result of this study was presented in the Fig. 3 from which we can see that the AN increased with the increased fermentation time. Also at the same time, the effect of salinity on the AN value was indicated from the figure. In a previous study, it was found that high salinity reduced the AN compared with the low salinity in the Jeotgal fermentation (32). In our study, the Jeotgal added with 13% of salt released higher level of AN than all other Jeotgal groups during the 20 and 40 days. And at the end of the fermentation, Jeotgal added with 25% of salt showed the lowest AN compared with the other groups. The difference of AN may also be attributable to the different salinity, but the exactly mechanism need further study and investigation.
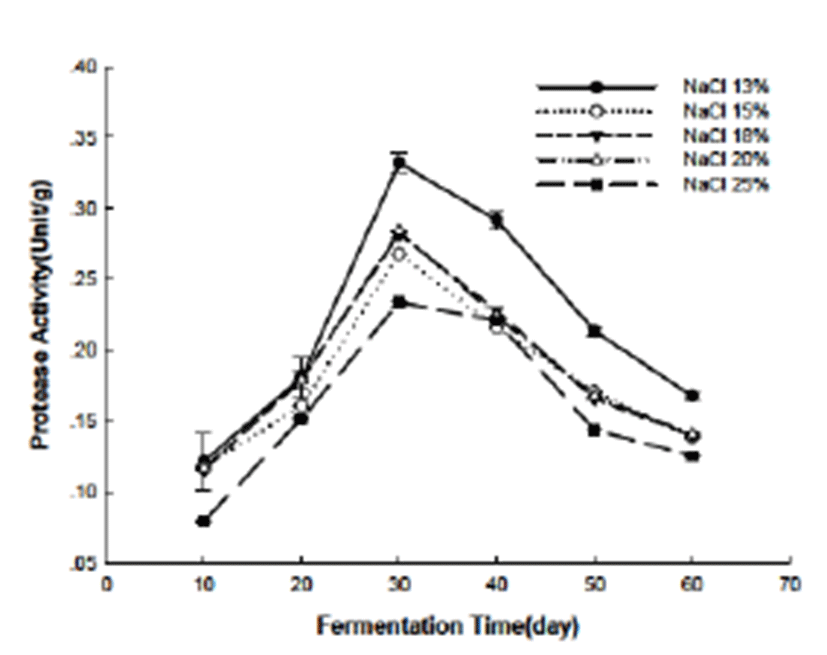
During the fermentation, the proteases which would degrade the protein to peptides and amino acids were derived from two sources: the abalone viscera and the microorganisms. The protease activity (showed in Fig. 4) in this study indicated both the influence of the fermentation time and the salt concentration. As mentioned above, salt concentration may have impact on the growth of the microorganisms in the Jeotgal and such influence the protease produced by them indirectly. The results showed that higher salt concentration inhibited the protease activity compared with the Jeotgal added with lower salt concentration. We deduced that the lower number of the microorganisms and the changed conformation of the enzymes as the results of the higher salt concentration may lead to the lower protease activity. Moreover, the change of the protease activity suggested the influence of the fermentation time to the protease activity or the number of microorganisms in the fermentation. At the beginning of the fermentation, the microorganism number was much lower than the latter, at the 30th days for instance. At the 30th days the protease activity reached the highest. After that, the protease activity decreased accordingly. The change of the protease activity is related to the amino nitrogen and other flavor substance as well.
As have been mentioned in the previous study (24), abalone viscera contained significant amount of free amino acids which conferred to abalone special flavor. We investigated and compared the free amino acids composition of abalone viscera and the abalone viscera Jeotgal at the end of the fermentation. The result was listed in the Table 2. Total free amino acids amount of abalone viscera was 30.37 mg/g which was less than half of that of the abalone viscera Jeotgal. Except for the taurine, β-alanine, DL-3-aminoisobutyric acid, γ-aminobutyric acid, DL-plus allo-δ-hydroxylysine, Lornithine and L-anserine, all other free amino acids level in the abalone viscera Jeotgal was higher than the abalone viscera. Also in the abalone viscera Jeotgal, we detected L-3-methylhistidine which was not detected in the abalone viscera.
In this study, the customer responses to the abalone viscera Jeotgal and three other Jeotgal used as control groups were investigated. Sensory evaluation result was listed in Table 3. The changran Jeotgal got the highest score in the color evaluation which was followed by abalone viscera Jeotgal, oyster Jeotgal and abalone meat Jeotgal. In this study, costumers showed similar response to the color of oyster Jeotgal and abalone viscera Jeotgal and significant bad perception for abalone meat Jeotgal. In the taste test the changran Jeotgal received the highest score which was followed by abalone meat Jeotgal and abalone viscera Jeotgal and the oyster Jeotgal got the lowest score. However, the scores for abalone viscera Jeotgal (2.85±0.93) and abalone meat Jeotgal (3.35±1.04) was not significant different. The abalone viscera Jeotgal presented inferior score compared with the abalone meat Jeotgal and changran Jeotgal. As to the overall acceptability score, changran Jeotgal got the highest score and followed by abalone viscera Jeotgal, abalone meat Jeotgal and oyster Jeotgal. Changran Jeotgal is generally considered as the superior than other Jeotgal in Korean cuisine (33) and this is also confirmed by the sensory evaluation conducted in this study. Abalone viscera Jeotgal showed higher score in the color and overall acceptability evaluation but lower score in the taste and flavor tests which indicated the possible likeness of the costumers.
Conclusion
Jeotgal is a fermented seafood and special dish in Korean cuisine. In this study we proposed Jeotgal prepared with the abalone viscera and investigated its physicochemical properties. The proximate composition of abalone viscera was analyzed and the results suggested that it is highly nutritious and suitable for fermentation. The pH change during the fermentation indicated that pH decline along with the fermentation but both the pH change and influence of salinity is not considered as significant. Furthermore, we also studied the change of VBN and AN which all reflect the influence of fermentation time. VBN result showed the influence of the different salinity as Jeotgal added with higher amount of salt released lower VBN than that of Jeotgal added with lower amount of salt. We see both the VBN and AN increased along with the fermentation. However, the influence of salinity on AN variation was not that clear. Jeotgal added with 25% of salt revealed lower AN at the end of the fermentation than other Jeotgal groups while Jeotgal added with 18% of salt presented the highest AN value. The protease activity change during the fermentation indicated both the salinity and fermentation process. In the middle of the fermentation (30 day) the protease activity of all Jeotgal groups reached highest. Also, Jeotgal fermented with the lowest salinity showed the highest protease activity while the Jeotgal with the highest salinity showed the lowest. We examined the free amino acids both in the abalone viscera and abalone viscera Jeotgal at the end of the fermentation. Seven kinds of free amino acids in the abalone viscera Jeotgal presented lower or the same quantity while the level of the rest 26 kinds of free amino acids in abalone viscera Jeotgal were higher. Moreover, the total amount of free amino acids in abalone viscera Jeotgal (62.75 mg/g) is more than twice of that of abalone viscera (30.37 mg/g). At last, the sensory evaluation was carried out. Results show that abalone viscera Jeotgal is more acceptable for the color and overall acceptability while need improvement on the taste and flavor. As a conclusion, we proposed the abalone viscera Jeotgal for the first time and researched its characteristics and hopefully it will provide the basis for future study.