1. Introduction
Consumers’ choice of freshly cut meat is a function of the meat’s color, freshness, texture, and flavor. However, maintaining these attributes remains a daunting challenge due to the inherent nature of fresh meat. Fresh meat is highly perishable due to its high moisture and protein content. The perishability of fresh meat is exacerbated by environmental factors like exposure to air, temperature fluctuations, and microbial contamination (Anas et al., 2019). Thus necessitating the requirement for protective measures during processing and storage to extend its shelf life. In response to these challenges, various innovative packaging solutions, including modified atmosphere packaging, have been developed and tested to preserve the quality attributes of freshly cut meat (Casalini et al., 2023; Dalla Rosa, 2019). Notably, high-oxygen-modified atmosphere packaging (80% O2 and 20% CO2) is widely employed in the retail meat market to maintain the vivid red color of meat (Musa, 2019). However, this approach presents drawbacks, including vulnerability to enzyme-facilitated lipid oxidation, leading to discoloration and off-flavors (Amaral et al., 2018) and adverse effects on meat texture quality.
In recent years, edible coatings have emerged as a promising alternative to modified-atmosphere packaging for preserving the quality and extending the shelf life of fresh-cut meat. Edible coatings create a protective barrier on the meat’s surface, controlling the exchange of water and gases (O2 and CO2) (Ananey-Obiri et al., 2018; Shellhammer and Krochta, 2018), thus mitigating the deteriorative effects due to microbial spoilage and oxidation-mediated color change respectively. According to Guerrero et al. (2015), oxidative deterioration in meat influences meat’s texture. These coatings also mitigate dehydration rates and increase storage life (Naskar et al., 2022). Notably, edible coatings provide an eco-friendly and safe substitute for chemical preservatives commonly utilized in meat processing.
Hybrid composite coatings formulated from starch and keratin are gradually gaining prominence as a potential substitute for petroleum-based packaging materials owing to their non-toxicity, renewability, eco-friendliness (Oluba et al., 2021a, 2021b). Combining keratin and starch into a hybrid composite enables leveraging the high gaseous barrier attribute of starch and moisture barrier attributes of keratin in one hybrid composite. Thus, keratin-starch hybrid coatings exhibit low moisture and gas permeability (Oluba et al., 2022). Furthermore, a recent study by Oluba et al. (2023) has shown the antioxidant activity of keratin-starch composite coatings against pro-oxidant radicals, such as 2,2'-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid and nitric oxide, in vitro, indicating their potential as packaging materials for preserving the freshness of fruits and vegetables (Oluba et al., 2023). However, the antimicrobial effects of keratin-starch coatings have not been reported in literatures, hence the need to incorporate polyphenolic-rich extracts with established antimicrobial compounds into the keratin-starch coating in this study. Furthermore, keratin-starch composites have demonstrated effectiveness as carriers for bioactive compounds with antioxidant and antifungal properties, enhancing the quality and health benefits of food preservation (Varghese et al., 2023).
Lately, the meat packing industry has shifted towards creating active packages that have antioxidant and/or antibacterial properties. These active packagings are designed to significantly extend the time fresh meat may be stored before spoiling. When developing active packaging techniques, it is preferable to employ natural compounds instead of synthetic food preservatives. This is because the usage of synthetic compounds is currently limited (Nguefack et al., 2009).
Previous research has demonstrated the rich phenolic content of avocado peel, highlighting its potent antioxidant and antimicrobial properties against foodborne pathogens (Kupnik et al., 2023; Sadiye, 2021). However, integrating avocado extract into composite coatings for extending the shelf life of perishable foods remains a relatively unexplored avenue. The application of functionalized composite coatings to meats and meat products using various methods, including dipping, spraying, casting, and individual wrapping (Kumar et al., 2023; Song et al., 2021), serves to retard microbial spoilage, prevent oxidative discoloration, and mitigate off-flavors (Bhadu et al., 2023). Moreover, these coatings provide a barrier against moisture loss and gas diffusion (de Jesús Salas-Méndez et al., 2019).
Avocado (Persea americana) peel, known for its rich phenolic compounds with established antioxidant and antimicrobial activities (Melgar et al., 2018; Sadiye, 2021), represents a sustainable biomass for extracting functional molecules with promising applications across industries, including food preservation, pharmaceuticals, and cosmetics. Recent studies by Figueroa et al. (2021) and Oluba et al. (2022) reported the extraction of phenolic compounds from avocado peel through microwave-assisted technology, underscoring its potential for food preservation applications. Furthermore, the reported antifungal activity of keratin starch hybrid composite coatings impregnated with avocado peel polyphenolic-rich extract against tomato fruits infected with fungal pathogens suggests further promising applications of this extract as a functional ingredient in food processing (Oluba et al., 2022). Therefore, the present study focuses on the innovative functionalization of a waste chicken-derived keratin-ginger starch composite coating with avocado peel polyphenolic-rich extract and to evaluate the potential effects of this novel composite coating on the quality attributes and shelf life of freshly cut beef, thus addressing critical aspects of perishability and food preservation.
2. Materials and methods
Reagents and chemicals including sodium metabisulfite, sodium hydroxide, sodium chloride, trichloro acetic acid, potassium ferricyanide, 2-thiobarbituric acid, 2,4-dinitrophenylhydrazine were procured from Sigma-Aldrich Ltd. (Gillingham, UK), peptone water and plate number agar were procured from Oxoid (Hampshire, UK), while Pseudomonas selective agar was from Merck (Darmstadt, Germany).
Freshly slaughtered beef was obtained directly at the point of slaughter, approximately at 7:30 am, and subsequently transported to the laboratory. The meat was promptly processed for the experiment.
Waste feathers were procured from Landmark University Commercial Farms, Omu-Aran, Nigeria.
Freshly harvested ginger rhizomes, avocado pear (Persea americana), and tomato fruits were purchased from Omu-Aran market, Nigeria. The plant materials were certified by a taxonomist in the Department of Plant Science at the University of Ilorin, Nigeria, where voucher specimens were deposited. All aspects of plant collection and utilization adhered to relevant guidelines.
Starch was extracted according to the method described by Oluba et al. (2023), with minor modifications. Peeled ginger rhizomes were cut into small pieces, steeped in 0.2% (w/v) sodium metabisulfite solution (1:2 w/v) for 10 min, and ground in distilled water with a kitchen blender (Preethi® Chefpro, Chennai, India) at a speed of 1 for 3 min. The resulting suspension was diluted thrice and filtered through a 60-mesh sieve. The starch slurry was allowed to settle for 4 h, separated, and washed thrice in distilled water. The starch precipitate was added to a mixture of sodium chloride solution (0.1 M) and toluene (1:0.1 v/v) and allowed to settle for 3 h. The final starch precipitate was rinsed thrice with distilled water and oven-dried at 50°C for 3 h.
Keratin was obtained from waste feathers according to the method previously described by Oluba et al. (2021a).
The polyphenolic-rich extract of avocado pear peel (APPPE) was obtained using the method originally proposed by Simić et al. (2016) and subsequently modified as detailed by Oluba et al. (2022).
As Oluba et al. (2022) outlined, the procedure was adhered to with slight modifications. A solution of chicken feather keratin (5% w/v) and gelatinized ginger starch (5% w/v) was prepared, following the method described by Oluba et al. (2021a). The keratin and starch solutions, in a ratio of 0.3:25 (v/v), were separately dispensed into five 150 mL beakers. Varying volumes of APPPE (0.0, 0.2, 0.6, and 1.0 mL, designated as K-SC, K-SC-APPPE0.2, K-SC-APPPE0.6, K-SC-APPPE1.0, respectively) were subsequently added to these solutions, following the experimental design.
Fresh beef was procured directly from the slaughterhouse on the day of the experiment. The beef samples were subsequently cut into pieces measuring 2.5 cm×2.5 cm×1 cm. These beef pieces were meticulously washed and rinsed with distilled water. Each beef sample (twenty-five pieces per treatment) was individually immersed in its respective coating solution, as outlined in Table 1. They were fully submerged to ensure complete coverage of the entire beef surface. Subsequently, these coated beef samples were placed in sterile polypropylene trays, air-dried at room temperature, and vacuum-packed within polyethylene bags. The packed beef samples were stored in a refrigerator at 4°C for 12 days. At specific intervals of 0, 3, 6, 9, and 12 days, five beef sample pieces from each treatment group were retrieved, homogenized separately, filtered, and the filtrates were stored at 4°C for further analysis.
CTRL, control; K-SC, waste feather keratin-ginger starch composite coating; K-SC-APPPE0.2, K-SC-APPPE0.6, and K-SC-APPPE1.0, waste feather keratin-ginger starch composite coating containing 0.2, 0.6 and 1.0 mL avocado peel polyphenolic-rich extract.
For pH determination, 10 mL of filtrate obtained from each beef sample piece was diluted with 50 mL of distilled water, and the pH of the resulting solution was assessed using an electronic pH meter (H12210 pH meter).
Metmyoglobin, representing the percentage of total myoglobin perceptible at the steak surface, was measured spectrophotometrically by assessing steak surface reflectance (SR) at 525 and 572 nm (Minolta CM-2002, Osaka, Japan) (Stewart et al., 1965). The maximum value of the ratios of (K/S)572 to (K/S)525, corresponding to 0% metmyoglobin, was established at the beginning of the experiment. The K and S values were the absorption and scattering coefficients determined using the Kubelka-Munk equation from reflectivity (R) data. After oxidizing the sample in 1% (w/v) solution of potassium ferricyanide, 100% metmyoglobin was obtained through the same process (Ledward, 1970). The average value for each steak was calculated based on five determinations.
The 2-thiobarbituric acid (TBA) method described by Pfalzgraf et al. (1995) was used to assess lipid oxidation. This analysis was performed in duplicate using 5 g of beef. TBARS levels were determined using a malondialdehyde (MDA) standard curve and expressed as mg.MDA.g-1 of beef.
Protein oxidation was evaluated by derivatizing total carbonyls with 2,4-dinitrophenylhydrazine, following the method detailed by Oliver et al. (1987). The results are presented as carbonyl concentrations (nM)/mg of protein.
After a 12-day treatment 5 g of beef samples were placed into a stomacher bag under aseptic conditions and homogenized with 45 mL of sterile 0.1% peptone water using a kitchen blender for 2 min at room temperature. An appropriate peptone aqueous solution (0.1%) was prepared for each sample, and 1 mL or 0.1 mL of the respective sample suspension was poured twice or spread onto selective agar plates for total counting. Total viable numbers were determined on plate number agar (Oxoid, UK) and incubated at 30°C for 48 hours. Pseudomonas spp. were counted for molds and yeasts using Pseudomonas selective agar (Merck, Germany) supplemented with a selective supplement (As mentioned earlier), previously prepared and incubated at 25°C for 48 hours. Microbiological data were logarithmically transformed to represent colony-forming units per gram beef weight (CFU/g) (Lorenzo et al., 2014).
Results are presented as mean values with associated standard deviations. Mean comparisons were conducted using Tukey’s test at a 95% confidence interval through analysis of variance (ANOVA). Graphs were generated using GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA).
3. Results and discussion
The application of potent antioxidants is a common strategy in the meat industry to extend the shelf life of meat products (Makahleh et al., 2015). However, the concerns associated with synthetic antioxidants have led to the search for safer and more effective alternatives. Therefore, APPPE with reported antioxidant (Oluba et al., 2023) and antifungal (Oluba et al., 2022) activities remains a promising candidate for functionalizing edible composite for food packaging applications. Thus, this study was designed to evaluate the effectiveness of K-SC-APPPE coating in preserving the quality attributes (pH, color, flavor, and odor) in determining the shelf life of freshly cut beef stored at 4°C for 12 days.
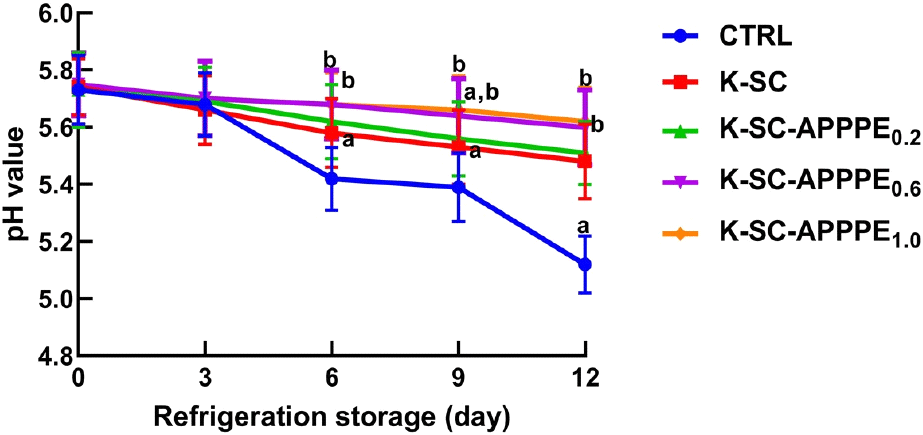
The pH value of the freshly cut beef, whether treated with a K-SC composite impregnated with or without APPPE, was not statistically different (p<0.05) from the control. However, as the storage period extended from 0 to 12 days, the pH of the control beef decreased from 5.73 to 5.09. Notably, this decline was statistically significant (p>0.05) when compared to beef coated with K-SC-APPPE composite, particularly at concentrations of 0.6 (K-SC-APPPE0.6) and 1.0 mL (K-SC-APPPE1.0) (Fig. 1). As observed in this study, the typical decline in pH attributable to lactic acid fermentation in meat spoilage was inhibited in freshly cut beef coated with K-SC-APPPE and stored for 12 days at 4°C. This is suggestive of the retardation in microbial activity vis-à-vis through the suppression of microbial growth (as revealed in this study) or that the inclusion of APPPE in the K-SC coating effectively restrained the proliferation of lactic acid bacteria or other microorganisms, thus preventing the acidification of the media through microbial fermentation. In agreement with the report of Gaikwad et al. (2020), the storage of beef in the control group at 4°C for 12 days in this study resulted in decreased pH. Additionally, reports from previous studies have shown that the inclusion of lactic acid bacteria may result in a decrease in the pH of packaged meat (Gaikwad et al., 2020; Hanani et al., 2022; Savoia et al., 2019; Sirocchi et al., 2017; Zahid et al., 2020). The observed pH values in the K-SC-APPPE-coated beef in this investigation fell within the standard range of 5.40-5.80. Contrary to the findings of this study, Chaari et al. (2022) reported a decrease in the pH of beef coated with gelatin-sodium alginate film containing beetroot peel extract for 14 days. pH is a crucial indicator of food stability, and it is frequently influenced by microbial and chemical interactions, which can contribute to food deterioration. The accumulation of organic acids, particularly lactic acid, resulting from microbial fermentation (Pellissery et al., 2020), is responsible for the observed decrease in pH during meat storage, as confirmed by a previous study (Casaburi et al., 2015; Fidan et al., 2020; Khodayari et al., 2019).
Consumers heavily rely on meat color as an indicator of freshness and safety, making it a critical factor in their purchasing decisions. Myoglobin, an iron-containing protein that gives meat its color, is primarily responsible for determining the oxidation state of meat (Faustman et al., 2023). In this study, the percentage metmyoglobin concentration in beef coated with K-SC-APPPE0.6 and K-SC-APPPE1.0 was observed to decrease by 28% and 26%, respectively, in comparison to beef coated with distilled water (i.e., control) after 12 days storage at 4°C, (Fig. 2). Conversely, metmyoglobin levels exhibited no significant change (p<0.05) between K-SC and the control groups. The observed change in metmyoglobin concentration in this study is in line with findings from previous studies suggesting that natural antioxidants, whether incorporated directly or indirectly into composite packaging, can significantly reduce metmyoglobin synthesis (Camo et al., 2011; Navikaite-Snipaitiene et al., 2018). Meat color, quality, and shelf life are significantly impacted by the oxygen levels in the packaging. The reduced oxygen concentration in the K-SC-APPPE-coated beef limits the oxidation of myoglobin and the susceptibility of the other beef components to oxidation. Thus, the generation of metmyoglobin, hydropereoxides, and other oxidative products could alter the organoleptic properties of the beef, including color, odor, and taste, and reduce its nutritional value (Wang et al., 2022). Due to the antioxidant property of APPPE, it can retard the oxidation of myoglobin to metmyoglobin, leading to longer maintenance of meat redness and freshness. In addition, APPPE may contribute to the UV-barrier capacity of the K-SC biocomposite, hence, serving to inhibit the susceptibility of the beef samples to photo-catalytic mediated degradation or changes in the color of quality (Elhadef et al., 2024). Oxidation byproducts, primarily from lipid and protein oxidation, can also have harmful effects on humans.
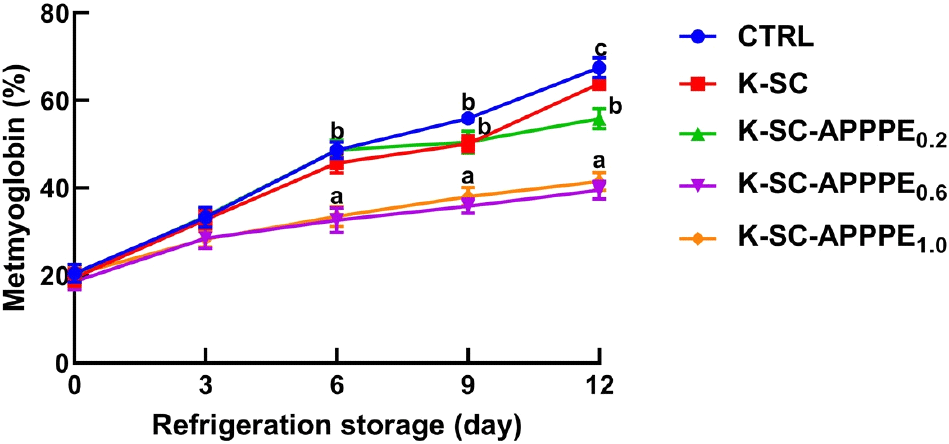
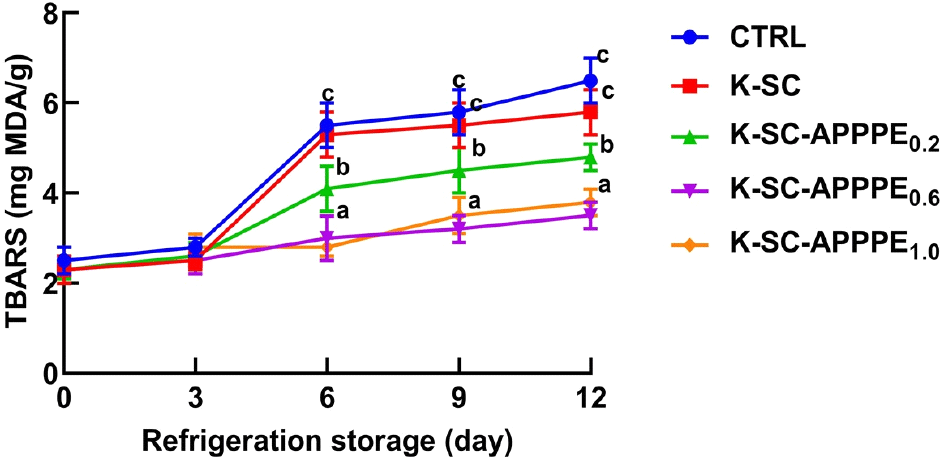
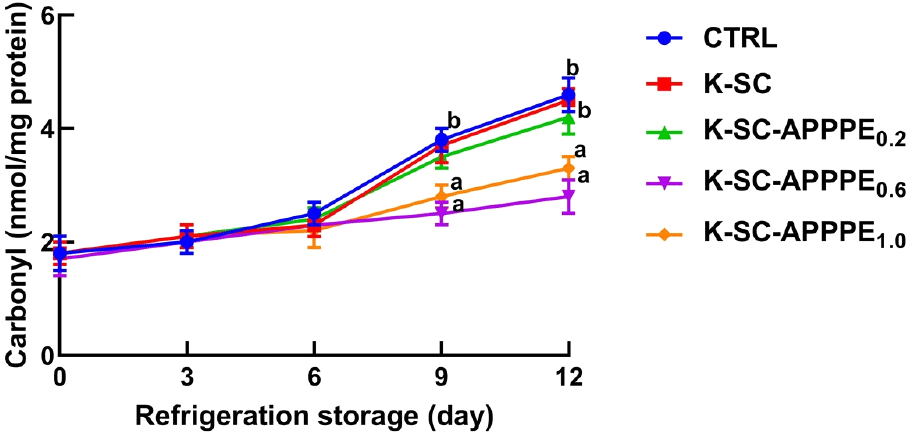
Fig. 3 illustrates the thiobarbituric acid reactive species (TBARS) values, which reflect the levels of secondary products resulting from lipid oxidation. The results indicate a significant (p<0.05) increase in TBARS levels in beef coated with the control, and K-SC and K-SC-APPPE0.2 as the storage duration extended. However, the alterations in TBARS concentrations on days 0 and 12 did not show statistical significance in beef coated with K-SC-APPPE0.6 and K-SC-APPPE1.0. Consequently, the incorporation of 0.6 and 1.0 mL of APPPE into the K-SC composite significantly inhibited the generation of the lipid oxidation product, TBARS, in freshly cut beef stored at 4°C for 12 days. Protein carbonyl levels in beef serve as a reliable indicator of protein oxidation and beef quality deterioration during storage periods (Choi et al., 2011). In Fig. 4, the effect of APPPE enriched-K-SC coatings on freshly cut beef stored at 4°C for 12 days concerning the generation of protein carbonyl concentration is illustrated. The results reveal that the application of K-SC, compared to the control, did not produce a statistically significant (p<0.05) effect on the protein carbonyl content. However, after 12-day storage period at 4°C, there was a significant reduction in protein carbonyl content in beef coated with K-SC-APPPE0.6 and K-SC-APPPE1.0 compared to the control. The incorporation of APPPE, particularly at concentrations of 0.6 and 1.0 mL, into K-SC composite coatings exhibited a significant capability to prevent or mitigate lipid and protein oxidation in freshly cut beef in this study. Similar to our findings, low levels of protein carbonyls were reported in meat packed in films containing natural extracts such as clove (Syzygium aromaticum) (Sánchez-Velázquez et al., 2022), cinnamon (Cinnamomum cassia), and Terminalia arjuna (Chauhan et al., 2019). In addition, Permal et al. (2020) demonstrated the effectiveness of avocado peel powder as an alternate antioxidant additive in inhibiting lipid oxidation in pork sausages. The K-SC-APPPE limits the exposure of the beef samples to oxygen and prevents the formation of lipid peroxide radicals via auto-oxidation. In addition, the phenolic compounds in the APPPE could also prevent the oxidation of lipids and protein components in beef by scavenging free radicals and or as electron donors (antioxidants), thus scavenging potential free radicals generated. The antioxidant action of phenolic compounds is mediated through their numerous hydroxyl groups and conjugated bond systems. The lipid and protein oxidation processes are notable contributors to the deterioration of meat quality, resulting in issues such as rancidity, off-flavors, undesirable taste, and alterations in meat color, texture, and nutritional properties. In a study conducted by Biswas et al. (2012), it was observed that applying ethanolic and aqueous extracts of curry leaves and mint leaves to raw pork meat decreased pH. Furthermore, this treatment effectively hindered the formation of oxidative products and reduced TBARS levels in the meat, particularly when compared to salt treatment during refrigerated storage. The antioxidative effects of polyphenols in this context are mediated by their capacity to function as scavengers of free radicals, inhibit lipoxygenase activity, and serve as reducing agents for metmyoglobin.
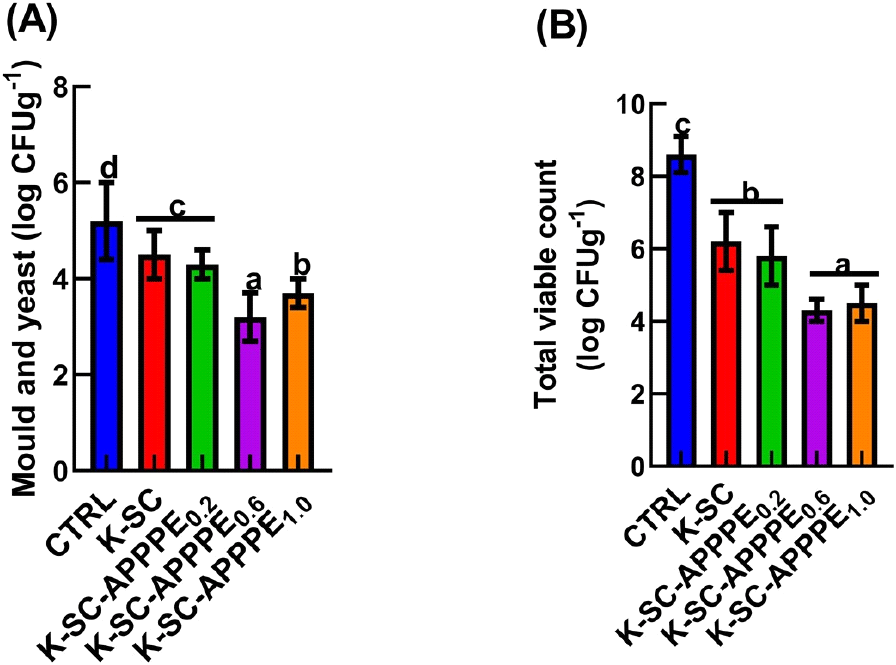
The mold and yeast population count within the various treatment groups fell within 2.8-5 log CFUg−1 range. Notably, beef coated with K-SC-APPPE0.6 exhibited a 45% reduction in mold and yeast populations compared with control. Similarly, meat coated with K-SC-APPPE1.0 experienced a reduction of 33% (Fig. 5(A)) in mold and yeast populations. Furthermore, the total microbial count was reduced by 50% and 48% in beef coated with the K-SC-APPPE0.6 and K-SC-APPPE1.0, respectively, compared with the control (Fig. 5(B)). As the storage period increased, the counts of molds and yeasts, and the total microbial count also increased. However, after a 12-day storage period, the treatment with avocado peel polyphenolic extract-enriched keratin-starch coating reduced microbial growth and final counts. These findings align with earlier research demonstrating the antimicrobial effects of natural extracts and compounds, supporting their use in food preservation (An et al., 2004; Arana-Sánchez et al., 2010; Biswas et al., 2015). Since fungi are aerobic organisms and cannot survive in the anaerobic environment that the coating on the meat creates, the reduced growth of yeast and mold in the samples coated with K-SC-APPPE may be due to the coatings’ oxygen barrier properties (Behbahani and Imani Fooladi, 2018). This is because the phenolic compounds in APPPE can disrupt the phospholipid bilayer of the cell membrane, letting more key compounds inside the cell leak out or stopping microbial enzyme systems from working normally (Ojagh et al., 2010). Avocado polyphenol, through its bacteriostatic effects, has been shown to retard the growth of gram-positive bacteria through their interaction with the bacterium cell membrane, thus disrupting bacterial protein and nucleic acid synthesis (Escalona-Arranz et al., 2013; Shi, 2023). Similarly, the study conducted by Lozano et al. (2017) revealed that chitosan-starch film containing beetroot extract displayed significant antimicrobial activity against mesophilic aerobic bacteria, coliforms, and fungi.
4. Conclusions
The results of this study indicate that K-SC-APPPE composite has a preservative effect on meat stored at 4°C, effectively preventing the oxidation of myoglobin to metmyoglobin as well as inhibiting potential auto-oxidation of lipids and protein components of beef into lipoperoxides (TBARS) and protein carbonyls, respectively. In addition, coating with K-SC-APPPE retards the growth of yeast and molds and subsequently suppresses the total microbial counts in beef stored at 4°C for 12 days. These preservative actions of K-SC-APPPE are attributed majorly to the antioxidant and antimicrobial properties of avocado peel polyphenols and the moisture and barrier properties of K- SC-APPPE. The results obtained in this study hold significant promise for advancing sustainable and environmentally friendly food packaging techniques while also exploring the untapped potential of avocado peel as a functional ingredient in biocomposite coatings. The outcomes of this investigation thus contribute to the development of effective, eco-friendly packaging solutions in addition to providing a sustainable strategy of utilizing solid wastes from both poultry and avocado processing industries.
