1. Introduction
Plant products are commonly used as an alternative for the chemical food preservatives and the plant secondary metabolites compounds (such as polyphenol and flavonoid) are natural sources of antioxidants (Sowmya and Anandhi, 2020). Recently, interest in free radicals has increased as a major factor in the progression of degenerative diseases (Boussaada et al., 2008). The oxygenation damages caused by reactive oxygen species on lipids, proteins and nucleic acids occurs various long-standing illness including aging, cancer, cardiac disorder and diabetes etc. It has been recognized that synthetic antioxidants used in food industry including butylhydroxyanisole (BHA) and butylhydroxytoluene (BHT) required substituted with natural antioxidants (Chen et al., 2016; Li et al., 2008; Li et al., 2013). Plant bioactive compounds including carotenoids, flavonoids and other beneficent phytochemicals are rich sources of dietary antioxidants (Basanta et al., 2016). The Poaceae is a large and very diverse family with about 600 genera and between 7,500 and 10,000 species. It is cosmopolitan in distribution (Walters and Keil, 1988). Phragmitis communis is naturally growing on both banks of river, lakes, ponds and low wetlands around the world to efficacy keep the ecological balance (Zhou et al., 2020). Phragmitis japonica is distributed on similar habitat with that of P. communis, the difference between P. communis and P. japonica is that the sheath color is green and purple. The rhizome of P. communis has used in heat pattern with such symptoms as high fever and thirst and the decoction have known in vitro antimicrobial action (Bensky and Gamble, 1986). And the natural growing Phragmites species from sewage water treatment sites throughout Europe are possible to absorb the organic xenobiotics and herbicides from water (Schroder et al., 2008). In Korea, the rhizome of P. communis have been used as an oriental medicine resources and the sprouts are used as raw materials for tea. Recently, environmental risks make reducing the community of reed. However, P. japonica, which is morphologically and ecologically similar with P. communis, is not used at all. The present study aimed to compare the antioxidant capacity of leaf and rhizome from P. communis and P. japonica for using as food and pharmaceutical materials.
2. Materials and methods
The leaf and rhizome of P. communis and P. japonica was collected from natural community along Dongcheon stream near Suncheon Bay, Suncheon-city, Jellanam-do Province of Korea. The two provenances were collected in June 2016 and the leaf and rhizome was air-dried at room temperature for 14 days. The leaf and rhizome were pulverized using an electric mill. The solvents for extraction and fractionation were purchased from DAEJUNG Co. (Shiheung, Kyeonggi-do, Korea) and the other reagents used for experiment were purchased from Sigma Co. (St. Louis, MI, USA) and Becton, Dickinson and Company (Sparks, MD, USA).
The powdered leaf and rhizome of Phragmites communis and Phragmites japonica (100 g) was extracted with 1,000 mL ethanol for 24 h at room temperature. The extracts were partitioned with 500 mL of hexane, ether, ethyl acetate and water successively. All the fractions were evaporated to dryness in vacuo.
The scavenging activity of DPPH radical of fractions from P. communis and P. japonica was measured by the modified Blois method (Blois, 1958). 500 μg of each dried fraction was dissolved in 1 mL of ethanol. 10 μL of each fraction was mixed with 90 μL of 2.0×10−4M DPPH solution (in ethanol). The mixtures were shaken and kept at room temperature in dark for 30 min, and measured the absorbance at 517 nm using a microplate spectrophotometer reader (EL800, Bioteck, Woonsocket, RI, USA). The radical scavenging activity of each fraction was calculated by the following formula;
where AA is absorbance of blank sample and ABis absorbance of sample fraction solution. L-Ascorbic acid and BHT was used for reference.
ABTS radical test was carried out by the modified ABTS decolouration assay (Re et al., 1999). ABTS radical cation was prepared by reaction of ABTS stock solution (7 mM in water) with 2.45 mM potassium persulfate and the prepared solution was kept at 4°C in dark place for 12 hr. The ABTS solution was diluted in ethanol to absorbance of 0.70±0.02 at 734 nm. 50 μL of each fraction was mixed with 2 mL of the diluted ABTS solution and the absorbance was measured at 734 nm with a microplate spectrophotometer (Jaitak et al., 2010). The ABTS free radical scavenging activity of each sample extracts was calculated as the DPPH free radical scavenging activity.
The content of total polyphenol was quantified using the modified Folin-Denis method (Velioglu et al., 1998). 1 mg of sample fractions were dissolved with the 1 mL ethanol. 25 μL of sample fraction solution was added with 500 μL of Folin-Denis’ reagent (10% in distilled water). The mixture solution was kept at room temperature for 5 min and centrifuged at 1,200 rpm for 10 min, and the clear supernatant was collected. 0.1 mL of the supernatant was mixed with 0.75 mL of Folin-Denis’ reagent. After 5 min, 500 μL of sodium bicarbonate (7.5% in distilled water) was added and the mixed solution was kept at 30°C in dark place. The absorbance of test sample was measured at 725 nm using microplate spectrophotometer reader. A standard curve with gallate (100-1,000 μg/mL) was used for quantification and the content was presented as milligram gallate per gram of fraction (mg Gallate/g).
The total flavonoid content (TFC) of the fractions was measured according to Moreno et al. (2000), with slight modification. 10 μL of each sample fraction (1 mg/mL) was diluted with 80% aqueous ethanol (90 μl). An aliquot of 0.5 mL was added with 2 μL of 10% aluminum nitrate, 2 μL of 1 M aqueous potassium acetate, 86 μL of 80% ethanol, and the solution was kept at room temperature for 40 min. The absorbance was measured at 725 nm using a microplate spectrophotometer reader after 7 min. A standard curve with quercetin (100-1,000 μg/mL) was prepared for quantification and the content was displayed as milligram quercetin (QUE) per gram of fraction (mg QUE/g).
All tests were carried out in triplicate. Statistical analysis was performed with the SPSS software (Version 25.0, SPSS Inc., Chicago, IL, USA). The data was represented the mean±standard deviation. Statistical differences between means were tested through Duncan’s multiple range test. The correlation between ABTS and DPPH radical scavenging activities of the tested fractions and total polyphenol and flavonoid contents were established by Pearson’s method.
3. Results and discussion
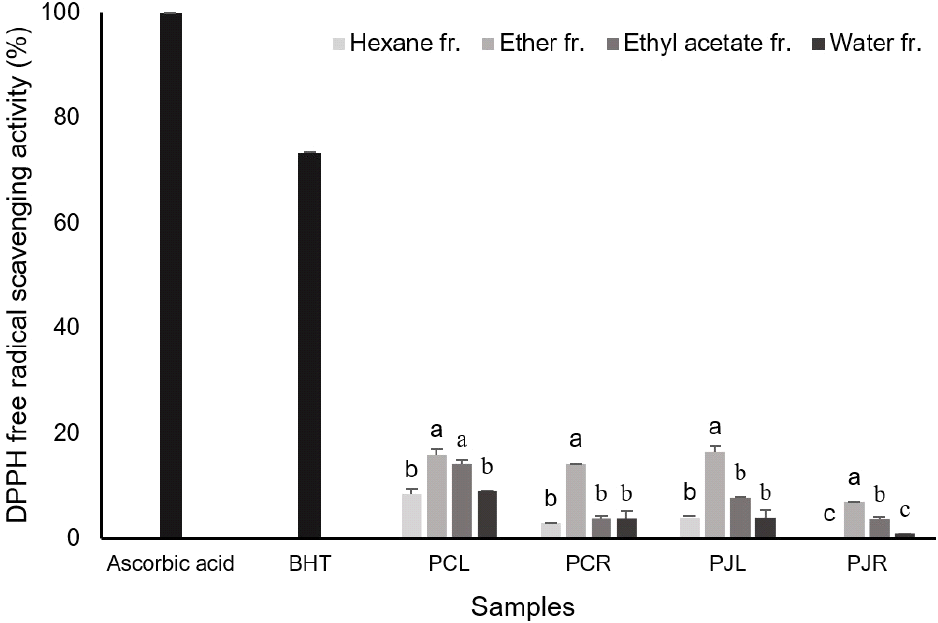
Different solvent-mediated successive fraction of leaf and rhizome of P. communis and P. japonica showed a varying DPPH free radical scavenging activity (Fig. 1). As that is known, scavenging activity (%) is closely related with the antioxidant effect of the solvent fraction. The ether fraction of the two Phragmites plant was shown to exhibit DPPH scavenging activity, which is lower than that of chemical synthetic antioxidant BHA. Li et al (2013) showed that the antioxidant capacities of 223 medicinal plants used most commonly in China were evaluated using ferric-reducing antioxidant power and Tolox equivalent antioxidant capacity assays. Among the tested plants, P. communis was shown to had lower antioxidant capacity. The radical scavenging activity of DPPH in the leaf fractions showed higher compared with the rhizome fractions. Lahmadi et al. (2019) showed that DPPH scavenging ability had a positive relation extract concentration and inhibition percentage of DPPH radical in the seed extract of Euphorbia retusa was higher compared with BHA, stem and leaf extract. The activity of various solvent extract methods and extraction procedure on the antioxidant ability of Potentilla atrosanguinea had been reported using ABTS radical scavenging activity, DPPH radical scavenging activity and FRAP assays (Kalia et al., 2008).
The ABTS scavenging reduction indicated that the inhibition percentage of the rhizome fractions was more than that of leaf fraction, it is quite similar results compared to those obtained in DPPH assay. The ether fraction of the two Phragmites plant showed higher ability to scavenging the ABTS radical than the other fractions. The ABTS radical scavenging activity of ether fractions from rhizome was not difference between P. communis and P. japonica (Fig. 2). As the results of the DPPH, ABTS radical scavenging assay, two Phragmites plant ether fractions were shown to significantly antioxidant ability. Jaitak et al. (2010) showed that butanol fraction of Potentilla fulgens was higher ABTS, DPPH radical scavenging activity comparable to the ethyl acetate and water fractions.
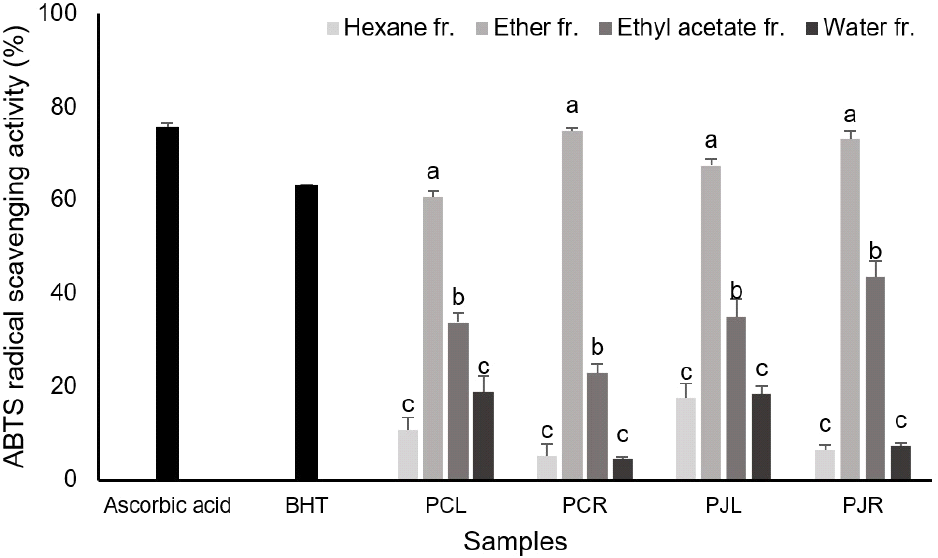
Table 1 shows the total polyphenol contents of the fractions from leaf and rhizome of the two Phragmites plants. Among the tested fractions, the ether fraction with the higher total polyphenol contents was leaf of P. communis (37.85±1.53 mg/g) and rhizome of P. communis (36.08±0.54 mg/g). The ether fraction of leaf and rhizome from P. communis had the higher DPPH and ABTS radical scavenging effect than other fractions. It was noticed that the leaf ethyl acetate fraction and rhizome hexane fraction of P. japonica showed higher total polyphenol contents than other fractions. Phenolic components are plant metabolite characterized by the presence of various phenol groups. The phenolic compounds had strong reactive in neutralizing free radicals by donating a hydrogen or an electron (Li et al., 2013) and it was reported that plant polyphenolic compounds including strong antioxidant activity (Kahkonen et al., 1999). Previous studies have shown that polyphenol content of plant parts is affected by numerous environmental reasons such as genetic factors, age, and vegetation period (Hofman et al., 2020).
Flavonoids are one of the most abundant phenolic compounds in the plant flora, and the antioxidant ability of plant flavonoids showing can be attributed to their free radical scavenging effect. (Boussaada et al., 2008). Unlike total polyphenol contents, hexane fraction exhibited richer in total flavonoids content than other fractions (Table 2). And the leaf fractions showed higher total flavonoids contents as compared to the rhizome fractions. Results were comparable with the total flavonoids content (e.g., leaf of P. communis: 42.44±1.60 mg/mL, rhizome of P. communis: 1.92± 0.23 mg/mL, leaf of P. japonica: 42.21±1.79 mg/mL, rhizome of P. japonica: 2.15±0.40 mg/mL for hexane fractions respectively). As a result of this study, proving the findings indicating that the presence of plant metabolites possible to variation depending on plant parts (Karoune et al., 2015) and sample treatment and extraction condition (Rumbaoa et al., 2009).
Generally, plant extracts with a high antioxidant effect had a high polyphenol content as well. Therefore, correlation coefficient (r) was calculated for estimate the correlation with TPC, TFC and antioxidant activity (Khaled-Khodja et al., 2014). In this study, the positive correlation between ABTS radical scavenging activity and TPC (r=0.824, p<0.05) is shown (Table 3). There is no correlation between ABTS radical scavenging activity and TFC (r=0.072). Negative correlation is shown between TPC and TFC (r=−0.123). Deng et al. (2015) showed highly significant relationships between DPPH and TPC and TFC and many components, including flavonoids and phenolic compounds are distributed in various plant organs, where they contribute important vital in the antioxidant activity. And Ouerghemmi et al. (2017) showed that DPPH radical scavenging activity increased proportionally to the flavonoid content (r=0.99).
| Factor | ABTS | DPPH | TPC | TFC |
|---|---|---|---|---|
| ABTS | 1 | |||
| DPPH | 0.824**1) | 1 | ||
| TPC | 0.567* | 0.421* | 1 | |
| TFC | 0.072NS2) | 0.423* | −0.123 | 1 |
4. Conclusions
This is the first study on the antioxidant capacity and total polyphenol and flavonoid content of P. communis and P. japonica, which are used as reeds. The fractions of leaf extract from the two Phragmites plants (4.06±1.32-16.47±1.28%) showed higher antioxidant activity by DPPH assay when compared with rhizome fractions of two Phragmites plants (0.00±0.00-14.15±0.07%), these are lower compared with ascorbic acid and butylhydroxyanisole (BHA). The highest ABTS radical scavenging activity was found for rhizome ether fraction, namely 74.95± 0.56% and 73.04±1.85% for P. communis and P. japonica, these are higher than BHA. The results from these antioxidant tests, such as DPPH free radical scavenging and ABTS radical scavenging activity and total polyphenol and total flavonoids contents, demonstrated that phytochemicals in P. communis and P. japonica have effects on antioxidant capacity. Overall, the extract solvents and plant parts affect the antioxidant property, total polyphenol contents and total flavonoids content, but it was not shown the difference in antioxidant activity between the two Phragmites plants. These results highlighted the potential of the P. japonica can be a resource of extracts as natural antioxidative for food and pharmaceutical applications like P. communis.
