1. Introduction
Panax ginseng is a perennial herbaceous plant of the Panax genus in the Araliaceae family, distributed in East Asia, North Siberia, and North America. Various species of the Panax plants grow naturally worldwide, and some are cultivated to use as medicine or food materials. At present, the most commonly cultivated species of the Panax plants is Panax ginseng C. A. Meyer. P. ginseng has long been used as an herbal material in oriental medicine and an ingredient of health supplements or functional foods in the food industry. P. ginseng is the most frequently consumed as medicinal plant resource (Cho et al., 2012; Park, 1996; Troung and Jeong, 2022). Several studies have investigated the bioactivities of the constituents of P. ginseng (including fresh ginseng, white ginseng, red ginseng, and their processed products), and among these, the most widely studied constituents are ginsenosides, a type of saponin. Additionally, the pharmacological effects of nitrogen-containing compounds (proteins, peptides, and amino acids), phenolic compounds, polysaccharides, polyacetylenes, and alkaloids have been explored, with their structures being identified. Various bioactivities have been identified for P. ginseng, including its effects on central nervous system disorders, memory, and learning; anti-stress, anticancer, immune-boosting, and antidiabetic effects; enhanced liver function, detoxification, anti-atherosclerotic, anti-cholesterol, and preventive effects on menopausal disorders and osteoporosis (Attele et al., 1999; Choi and Yang, 2012; Jee et al., 2014; Mancuso and Santangelo, 2017; Park, 1996).
Meanwhile, P. ginseng exhibits aroma characteristics different from other ginseng species, such as P. quinqefolium, P. notoginseng, and P. japonicum. According to literature, essential oils isolated from P. ginseng comprise diverse constituents with different chemical structures, which exhibit antioxidant activity (Bak et al., 2012; Chung et al., 2011), an ability to reduce the phototoxicity induced by oxidative stress and ultraviolet rays (Ullah et al., 2021), antidiabetic and lipid-metabolism-enhancing effects (Kim et al., 2011), hepatoprotective activity (Bak et al., 2012), an ability to improve the symptoms of Alzheimer’s disease (Kawamoto et al., 2019), and antimicrobial activity (Han and Park, 2008). Studies on the essential oil composition of P. ginseng have been ongoing since the 1980s, revealing that sesquiterpene and sesquiterpene alcohol compounds are abundant in P. ginseng (Kim and Park, 1984; Yoshihara and Hirose, 1975). Kim and Park (1984) isolated essential oil from fresh P. ginseng by steam distillation and analyzed chemical compositions of the oil using gas chromatography (GC). Approximately 250 peaks were detected, and 28 different constituents were identified through subsequent GC-mass spectrometry (MS) analyses. Most of unidentified constituents were presumed to be sesquiterpene and sesquiterpene alcohol compounds based on the mass spectral data. Later, follow-up studies identified several sesquiterpenoid compounds from solvent extracts of fresh P. ginseng such as ginsenol, spathulenol, 4β,10α-aromadendranediol, neointermedol, panasinsenols A and B, panaxene, panaginsene, ginsinsene, and so on. (Iwabuchi et al., 1987; Iwabuchi et al., 1988; Iwabuchi et al., 1989; Richter et al., 2005) and also the effects of the cultivation conditions and different Panax species on the essential oil composition have also been studied. (Cho et al., 2012; Kim et al., 2023; Ko et al., 1996). Although the essential oil in P. ginseng contain abundant sesquiterpenoid compounds, these constituents are not responsible for the unique aroma of fresh P. ginseng. Moreover, the aroma intensity is too low for these constituents to generate the characteristic aroma of P. ginseng. On this basis, Iwabuchi et al. (1984) obtained neutral, basic, acidic, phenolic, and aldehydic fractions of the solvent extract of dried P. ginseng (white ginseng) and identified 13 pyrazine compounds from the basic fraction that had the characteristic aroma of P. ginseng. However, no study has specifically identified the constituents of the phenolic and aldehydic fractions.
In addition, methoxypyrazine compounds have been reported as the constituents closely related to the characteristic aroma of P. ginseng, but they do not provide a sufficient explanation for the unique aroma properties of P. ginseng.
Hence, this study aimed to identify novel volatile constituents of the essential oils in P. ginseng. Steam distillation and solvent extraction method was used to isolate an essential oil condensate of from fresh P. ginseng. The essential oil divided into neutral, basic, acidic, phenolic, and aldehydic fractions by solvent partitioning. Several new constituents of P. ginseng that had not been previously discovered were identified through instrument-based analysis.
2. Materials and methods
The fresh P. ginseng samples used in this study were six-year-old P. ginseng roots cultivated in Jinan-gun, Jeollabuk-do, Korea, and harvested in late October 2018. The roots were purchased from the Geumsan market. The samples were first washed in an ultrasonic cleaner (Model Schark 900, Gideonsonic Co., Hanam-si, Korea) to remove soil and other foreign substances and subsequently under running water. The stable P. ginseng roots alone were used as samples.
The reference materials for the comparison of Kovat’s retention index (RI) with those of the constituents isolated by GC were monoterpenes, sesquiterpenes, and an n-alkane standard mixture (C8-C30) those were purchased from Fluka Chimie AG (Spruce St., St. Louis, MO, USA) and Sigma-Aldrich (St. Louis, MO, USA) and pyrazines purchased from Pyrazine Specialty, Inc. (Ellenwood, GA, USA). dichloromethane and diethyl ether of high-performance-liquid-chromatography grade were purchased from J. T. Baker (Avantor Performance Materials, LLC, Radnor, PA, USA). Other analytical-grade reagents were purchased from Daihan Scientific Co., Ltd (Wonju-si, Korea).
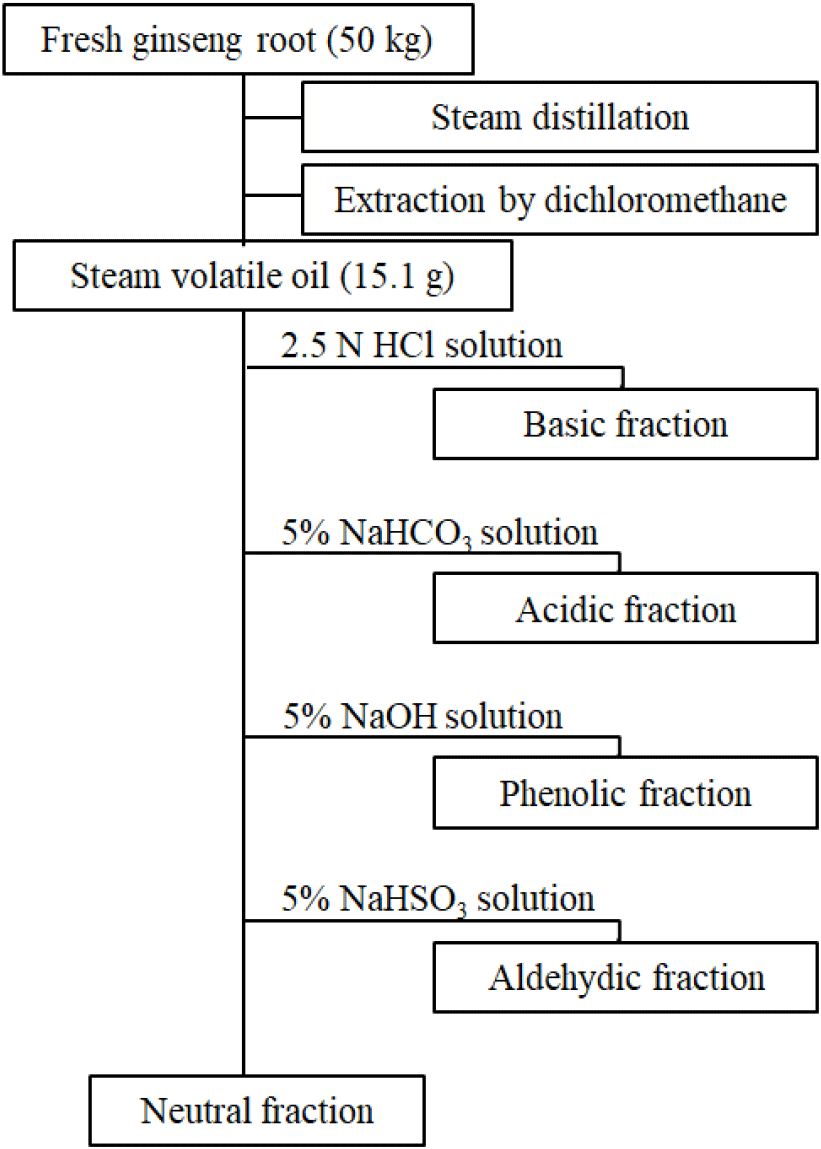
To isolate the essential oils from fresh P. ginseng, a cooling tube was connected to a commercially available ginseng steamer (76×75×108 cm, Daewon General Machinery Co., Jincheon, Chungbuk, Korea). A mixture of 50 kg of the fresh P. ginseng root and 10 L of water was placed in the steamer. With slight modifications to the method adopted by Kim and Park (1984), the steam produced during the 3 h distillation at 96-98°C was cooled at 4°C to obtain 4 L of condensate. The condensate was divided into 2 L portions for subsequent extraction using dichloromethane (1 L×3 times). The extracts were concentrated in a rotary evaporator at ≤40°C, and essential oils with the unique aroma of fresh P. ginseng were obtained. After weighing the essential oils, 300 mL of diethyl ether was used to dissolve the oils. Subsequently, according to the method described in Fig. 1, the neutral, basic, acidic, phenolic, and aldehydic fractions were obtained (Iwabuchi et al., 1984; Kim and Park, 1984; Kubeczka, 1984).
A 6890 gas chromatograph of Agilent Technologies (Santa Clara, CA, USA) was used for GC. The polar column was a Supelcowax 10 fused silica capillary column (30 m×0.32 mm, 0.25 μm, Supelco, Inc., Bellefonte, PA, USA). The column temperature was raised at 2°C per min from 50°C to 230°C; the temperature of 230°C was maintained for 20 min. The inlet and detector temperatures were set at 250°C, respectively. Nitrogen gas was used as the carrier; the flow rate was 1.2 mL per min, and the analysis was performed in the split mode (split ratio=30:1). A GC–MS system connecting the Agilent 6890N GC and the Agilent 5975 mass selective detector (MSD) was used for GC–MS. The column and analysis conditions were identical to those for the GC analysis. Additionally, the following conditions were set for the MSD: ionization voltage at 70 eV, electron multiplier energy at 1,600 V, ion source temperature at 250°C, and mass scan range at 50-500 m/z. The mass spectra of GC–MS were used to identify each constituent. The Wiley/NIST (National Institute of Standards and Technology, US Department Commerce, Gaithersburg, MD, USA, USA) data library was searched, and the retention time was compared between the GC data and the NIST gas chromatographic retention webbook data (https://webbook.nist.gov/chemistry) or the reference material. A constituent identified solely by comparing the mass spectral data was considered tentatively identified. The linear RI was calculated based on the analysis of the n-alkane mixture (C6-C30) under conditions identical to those of the GC analysis (Van Den Dool and Kratz, 1957).
Phenylalkenal compounds were experimentally prepared in this study to elucidate the formation mechanism and identify the chemical structures of novel phenylalkenals in the aldehydic fraction. For this, 1.0 mL of phenylacetaldehyde and 0.5 mL of n-hexanal or n-heptanal were mixed with 25 mL of 0.1 N citrate-phosphate buffer (pH 5.0). The mixture was reacted at 100°C for 2 h (De Schutter et al., 2008). The reaction solution was then cooled to ambient temperature, followed by extraction using diethyl ether (50 mL×3 times). This was followed by drying using anhydrous sodium sulfate for 12 h and filtration. The filtrate was concentrated using a rotary evaporator and dissolved in 5 mL of acetone for the GC and GC–MS analyses. For the analyses, a non-polar column, DB-5MS capillary (30 m×0.32 mm, 0.25 μm, Agilent Technologies), was used under conditions identical to those for the analysis of essential oils using a polar column.
3. Results and discussion
When consumers purchase fresh P. ginseng or its processed products such as white and red ginsengs, its authenticity or quality is determined primarily through visual examination; nevertheless, its aroma through the olfactory sense is also critical in determining its purchase. The essential oils in P. ginseng have been recently demonstrated to confer the unique aroma properties of P. ginseng and comprise various bioactive compounds (Bak et al., 2012; Chung et al., 2011; Kawamoto et al., 2019; Truong and Jeong, 2022; Ullah et al., 2021). To define a more detailed composition of the essential oils in fresh P. ginseng to produce red ginseng using fresh P. ginseng roots, concentrates containing volatile constituents were obtained by cooling the steam generated during distillation under conditions similar to those for the steaming process. Fig. 2(A) presents the GC profile for the total essential oil obtained from P. ginseng root.
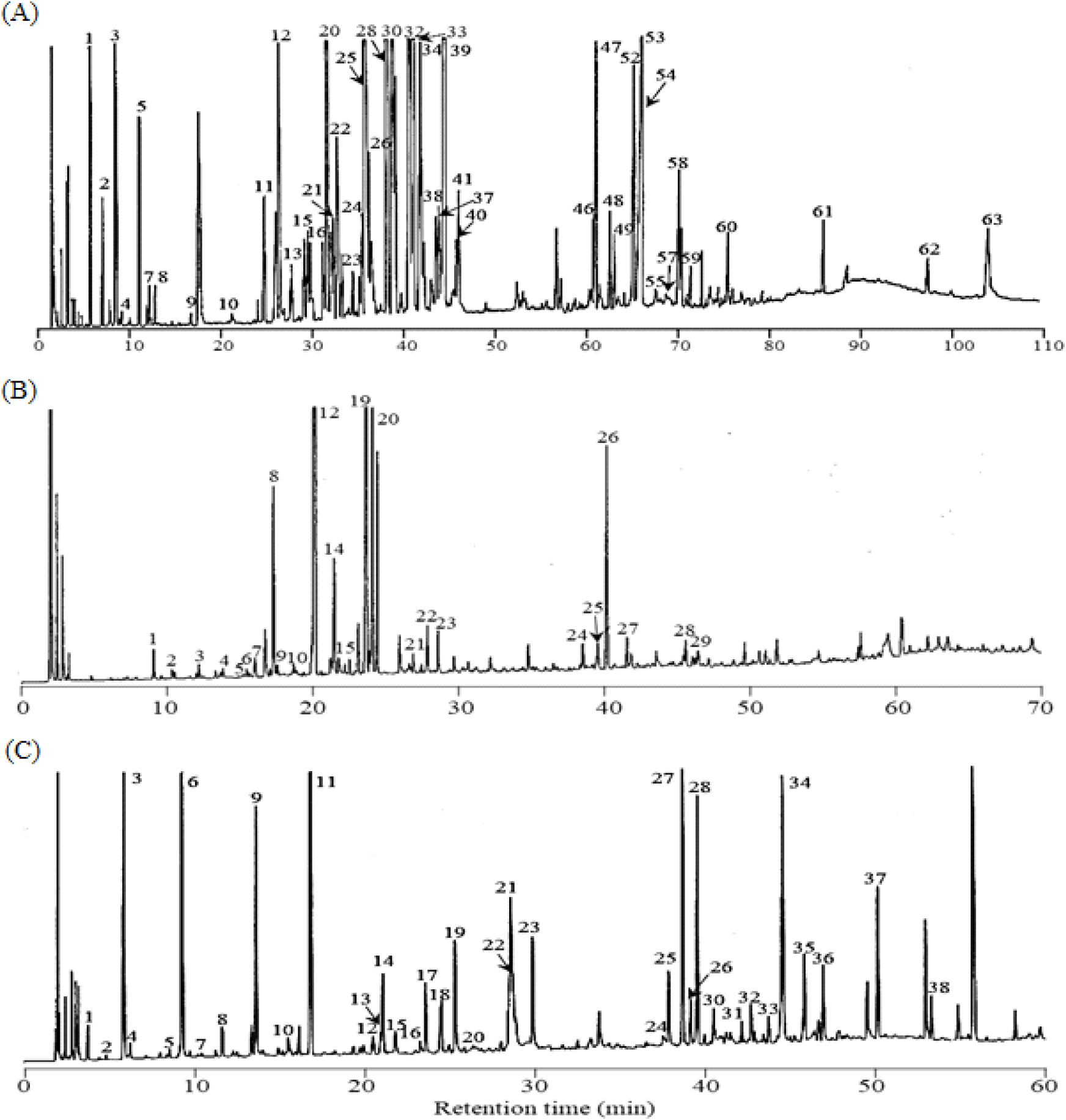
Table 1 presents the constituents identified by the GC–MS analysis of the total essential oil of fresh P. ginseng based on data library search, comparison of RI with the current literature values, and comparison of the retention time with GC data and reference materials. Sixty-three compounds were identified from the essential oils of fresh P. ginseng before fractionation. Excluding higher fatty acids and their esters, most of the identified compounds were terpenoids that had been previously identified from the essential oils of fresh, white, and red ginsengs (Cho et al., 2012; Kim and Park, 1984; Ko et al., 1996; Zhang et al., 1990). As shown in Table 1, monoterpene compounds (136 m/z), sesquiterpene compounds (204 m/z), and sesquiterpene alcohol compounds (222 m/z) were primarily detected in retention time ranges of 5-10 min, 25-45 min, and 60-70 min, respectively. The content ratio of the constituents revealed that the following compounds were present in high amounts: β-pinene (3.97%) and α-pinene (1.63%) for monoterpenes; α-humulene (13.91%), bicyclogermacrene (13.59%), β-caryophyllene (8.24%), α-neoclovene (7.78%), β-panasinsene (7.53%), and α-panasinsene (5.14%) for sesquiterpenes; and neointermedeol (2.20%), panasinsenol B (2.17%), ginsenol (1.69%), and spathulenol (1.65%) among the 14 compounds of sesquiterpene alcohols. While the 63 constituents identified in this study accounted for 97.23% of all the detected peaks, the level of their contribution to the characteristic aroma expression of fresh P. ginseng is known to be low despite its high contents (Iwabuchi et al., 1984).
Thus, for more detailed analyses of the essential oil composition of P. ginseng in this study, the essential oils were fractionated to obtain neutral, basic, acidic, phenolic, and aldehydic fractions, and the yield of each fraction was comparatively analyzed. The neutral fraction accounted for most of the content at 95.81%; the contributions of all other fractions were low, i.e., basic (0.55%), phenolic (1.10%), acidic (1.88%), and aldehydic (0.66%) fractions (Fig. 1). In the study of Kim and Park (1984), the essential oils in P. ginseng were fractionated similarly, and the aroma evaluation of each fraction indicated that the neutral fraction carried the unique aroma of P. ginseng. Conversely, Iwabuchi et al. (1984) reported the basic fraction to have the unique, strong aroma of P. ginseng. The result of this study agreed with the latter, whereby the neutral fraction was found to have a weak terpene or herbal-like aroma, and the basic fraction was found to have the strong characteristic aroma of fresh P. ginseng. Moreover, the aldehydic fraction had a weak, green, and camphor-like aroma; the phenolic fraction had a smoky and cresol-like aroma; and the acidic fraction had a rather pungent, cheese-like, and butyric-acid-like aroma. Per the GC and GC–MS analyses of each fraction, most constituents from the neutral fraction were monoterpene, sesquiterpene, and sesquiterpene alcohol compounds, and low-molecular-weight fatty acids were primarily detected in the acidic fraction. Therefore, this study excluded the neutral and acidic fractions, and only the basic, phenolic, and aldehydic fractions were used for the composition analysis.
Fig. 2(B) depicts the GC profile for the basic fraction. Through the GC and GC–MS analyses and by comparing the retention time with that of the reference material, 29 constituents were identified (Table 2). Most of these constituents were nitrogen-containing compounds. In quantitative terms, the contents of 2-isopropyl-3-methoxypyrazine (35.51%), 3-sec-butyl-2-methoxy-5-methylpyrazine (31.54%), 3-isobutyl-3-methoxypyrazine (8.64%), and 2-methoxy-3-methylpyrazine (8.40%) were high, in line with the results reported by Iwabuchi et al. (1984).
Table 3 presents the constituents identified in the phenolic fraction. As with other fractions, numerous peaks were detected in the phenolic fraction, but only 19 constituents were identified because the mass spectral data were unclear for the remaining compounds. The peak area of the 19 identified constituents was ~82.81%, with high amounts of benzoic (25.40%), octanoic (11.57%), nonanoic (9.16%), propionic (6.35%), decanoic (6.16%), heptanoic acids (5.12%), and eugenol (4.08%), with phenol (3.03%), salicylaldehyde (2.04%), guaiacol (1.74%), vanillin (1.44%), and 4-vinylguaiacol (1.28%), previously identified from the essential oils of P. ginseng (Han and Park, 2008).
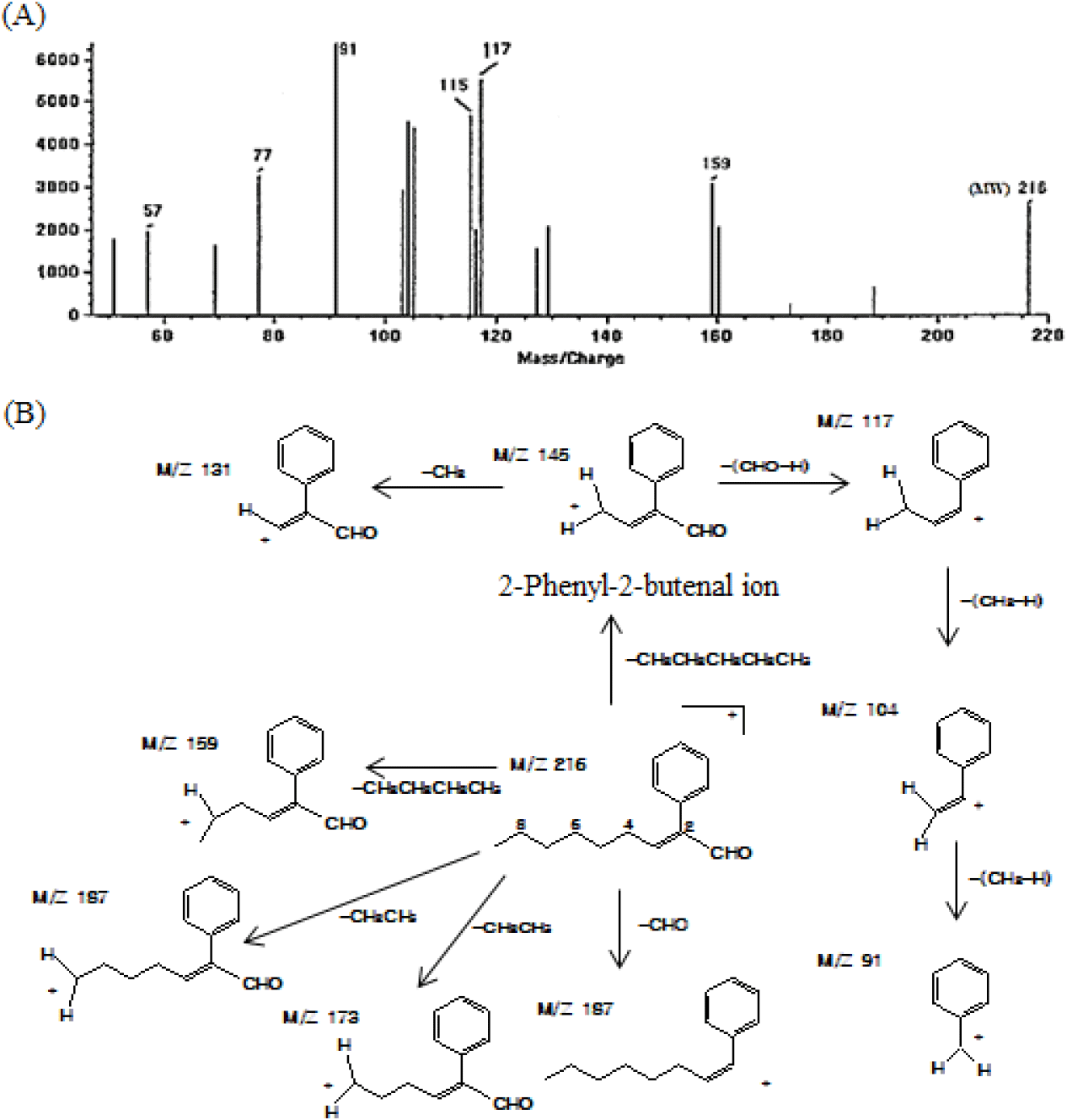
Notably, the aldehydic fraction accounts for only ~0.66% of the total essential oil; however, numerous peaks were detected in the GC profile, as shown in Fig. 2(C). Additionally, among the identified constituents, the contents of 4-(2-furyl)-2-buten-2-one (23.41%), benzaldehyde (10.18%), n-heptanal (9.42%), 3-(α-furyl) propenal (8.51%), 3-phenyl-2-butenal (7.28%), 2-furfural (7.10%), and trans,cis-2,4-heptadienal (4.62%) were high (Table 4). Multiple constituents were detected in the aldehydic fraction, attributed to both the compounds originating from fresh P. ginseng and those generated during steam distillation via the Maillard reaction and aldol condensation. For instance, the low-molecular-weight aldehydes among the identified constituents could be derived from fresh P. ginseng. These aldehydes are conjectured to contribute at least partially to the aroma generation of fresh P. ginseng owing to their fresh, spicy, and herbal-like aroma characteristics despite their low levels compared to those of monoterpenes or sesquiterpenes (Culleré et al., 2011; Plotto et al., 2004; Shui et al., 2019). Conversely, thiophene, furan, and alkenal compounds are likely to have been generated during distillation due to the Maillard and aldol condensation reaction. Notably, several unidentified phenylalkenal compounds were identified in the aldehydic fraction. These ingredients have not been previously reported in fresh ginseng and its processed products such as white ginseng or red ginseng. These novel constituents are the ones rarely detected in thermally processed foods. 2-Phenyl-2-butenal has been identified in malt wort and chocolates (Cha et al., 2019; Torres-Moreno et al., 2021). 2-Methyl-3-phenyl-2-propenal, 4-methyl-2-phenyl-2-pentenal, and 5-methyl-2-phenyl-2-hexenal have also been previously identified in processed foods, such as malt wort (Coghe et al., 2004; De Schutter et al.; 2008), cocoa powder (Bonvehí, 2005), and chocolate (Counet et al., 2002; Torres-Moreno et al., 2021). However, they were identified as constituents of the essential oils isolated from fresh P. ginseng for the first time. These constituents are presumed to have been generated when the essential oils were isolated from fresh P. ginseng through steam distillation, during which phenylacetaldehyde initiated an aldol condensation reaction with acetaldehyde, 2-methylpropanal, or 3-methylbutanal to produce 2-phenyl-2-butenal, 4-methyl-2-phenyl-2-pentenal, or 5-methyl-2-phenyl-2-hexenal, respectively (De Schutter et al., 2008). Additionally, the constituent (m/z 202) detected at the retention time of 49.89 min (peak 37) was identified as 2-phenyl-2-octenal based on the mass fragment pattern (De Schutter et al., 2008). This compound was also detected as a constituent of the essential oil from fresh P. ginseng for the first time. Notably, the constituent detected at the retention time of 53.03 min (peak 38) was highly similar in its mass fragment pattern to 2-phenyl-2-octenal, while its molecular mass was higher than that of 2-phenyl-2-octenal by m/z 14 (-CH2). The MS spectrum in Fig. 3(A) shows that the fragment ions (m/z 115 and 117) generated when an aldehyde group left the 2-phenyl-2-butenal ion from the basic structure were observed as the base peaks. Such fragment ions are commonly exhibited by the MS fragments of branched 2-alkenal compounds with a phenyl group, and they are presumed to be generated via pathways, as described in Fig. 3(B). Thus, based on the detected molecular ion peaks and patterns of the fragment ions, the chemical structure of the peak 38 was suggested to be 2-phenyl-2-nonenal, which is likely to be generated during the steam distillation via aldol condensation between phenylacetaldehyde and n-heptanal.
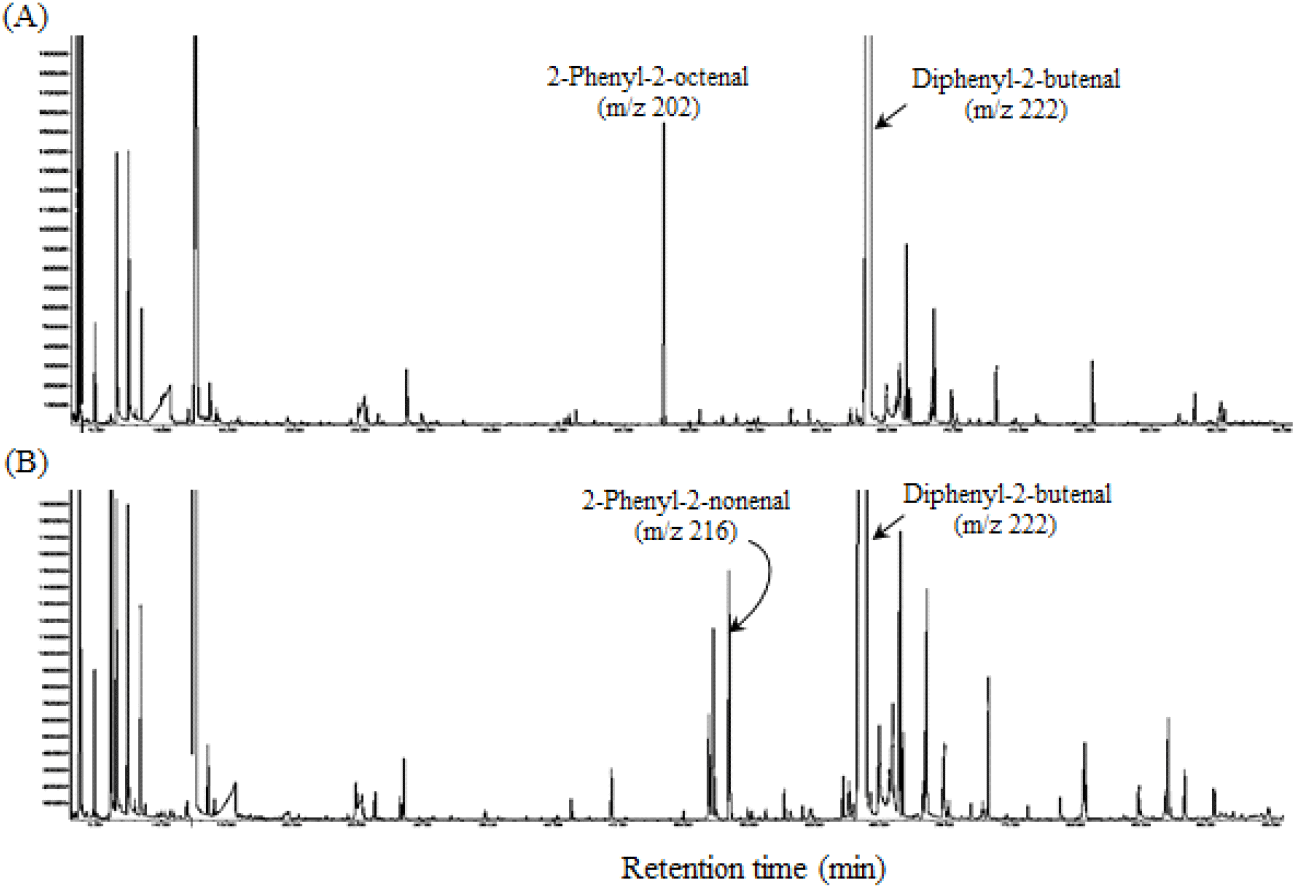
To prove this, a mixture of phenylacetaldehyde and n-hexanal or n-heptanal was heated under conditions similar to the steam distillation of P. ginseng. The result revealed that 2-phenyl-2-octenal (m/z 202) was detected in the reaction mixture of phenylacetaldehyde and n-hexanal (Fig. 4(A)), and 2-phenyl-2-nonenal (m/z 216) was detected in the reaction mixture of phenylacetaldehyde and n-heptanal (Fig. 4(B)). Based on these findings, the aldol condensation reaction of phenylacetaldehyde with n-hexanal or n-heptanal during steam distillation in fresh P. ginseng is conjectured to generate 2-phenyl-2-octenal and 2-phenyl-2-nonenal. Meanwhile, 2-phenyl-2-nonenal, in particular, is a new compound, unreported by any previous studies associated with plants or foods. Further studies should be performed to investigate the aroma properties of 2-phenyl-2-nonenal and its effects on the aroma characteristics of P. ginseng root.
4. Conclusions
The essential oil of fresh ginseng root was isolated by applying a steam distillation similar to the manufacturing process of red ginseng. After the oil was separated into neutral, basic, acidic, phenolic, and aldehydic fractions, Total essential oi, basic, phenolic and aldehydic fractions were analyzed by GC and GC–MS to determine the essential oil composition of fresh P. ginseng. Sixty-three compounds were identified in the total essential oil, and a majority of these were found to be monoterpenoids and sesquiterpenoids. In the basic fraction, the contents of 2-isopropyl-3-methoxypyrazine, 3-sec-butyl-2-methoxy-5-methylpyrazine, 2-isobutyl-3-methoxypyrazine, and 2-methoxy-3-methylpyrazine were high among the identified constituents. In the phenolic fraction, the contents of benzoic acid, eugenol, phenol, salicylaldehyde, guaiacol, and vanillin, as well as low-molecular-weight organic acids, were high. Among 38 compounds were identified in the aldehydic fraction, phenylalkenal compounds, including 5-methyl-2-phenyl-2-hexenal, 2-phenyl-2-octenal, and 2-phenyl-2-nonenal were newly identified in fresh P. ginseng and its processed products. These constituents are assumed to be generated by an aldol condensation that occurs between the phenylacetaldehyde and low-molecular weight n-alkanals during the steam distillation of fresh P. ginseng root.
