1. Introduction
Black soybeans, also known as medicinal beans or rice-mix beans in Korea, have a composition similar to common yellow soybeans and are high-protein, plant-based foods with physiologically active compounds (Kim et al., 2011). Notably, they have higher levels of sulfur-containing amino acids, such as methionine and cysteine, compared to red beans and mung beans (Koh et al., 1997). Black soybeans are also rich in lecithin, phytoestrogens, and unsaturated fatty acids, offering various pharmacological benefits such as anti-cancer, anti-obesity, and immune regulation properties (Jang et al., 2008; Kim et al., 2007; Liao et al., 2001). Moreover, trace amounts of β-glucan in black soybeans contribute to lowering blood cholesterol and triglycerides, enhancing the immune system and anti-inflammatory effects, and stimulating skin cell responses, which may delay aging (Lee and Bang, 2001). However, they also have a firm texture and contain substances such as trypsin inhibitors and volatile components that produce the typical beany flavor due to lipoxygenase. These issues can be mitigated by increasing digestibility and reducing off-flavor components through heat treatment, microbial fermentation, or enzyme treatment (Meinlschmidt et al., 2015).
Population aging is a pressing global concern. The UN World Population Prospects 2022 (United Nations, 2022) report highlights that, due to decreasing fertility rates and increasing life expectancy in developed countries, the global population, aged 65 and over, is expected to rise from 10% in 2022 to 16% by 2050. This trend is particularly pronounced in Korea, where the population’s aging is occurring at an extraordinary rate. According to the 2021 population projection released by Statistics Korea, the proportion of the population aged 65 and over is anticipated to reach 20.3% by 2025 and 46.4% by 2070 (Statistics Korea, 2021). However, a notable disparity exists between average life expectancy, which was 83.5 years in 2020, and healthy life expectancy, which stood at only 66.3 years. This gap has sparked increased interest in well-aging or healthy aging.
Aging involves a decline in tissue and cell functions and the degeneration of the nervous and musculoskeletal systems, which can lead to various age-related diseases (Guo et al., 2022). Among these, sarcopenia, characterized by reduced muscle mass and function, has traditionally been seen as a natural aspect of aging. However, it is increasingly being recognized as a disease worldwide. The USA assigned a specific disease code (M63.84) to sarcopenia in 2016, Japan recognized it as a disease in 2018, and Korea included it in the 8th revision of the Korean Classification of Diseases (KCD VIII), highlighting its global importance and risk (Cruz-Jentoft and Sayer, 2019). Sarcopenia results from various factors, including chronic inflammation, hormonal imbalances, nutritional deficiencies, and decreased physical activity (Gariballa and Alessa, 2013; Hara et al., 2016). Currently, there are no FDA-approved treatments for sarcopenia. Appetite stimulants such as megestrol acetate and synthetic anabolic steroids are sometimes prescribed, but their use requires caution due to potential side effects (Morley et al., 2014).
To prevent or mitigate sarcopenia, increasing physical activity, muscle strength training, and high protein intake are recommended strategies. Clinically, the consumption of high-protein foods, such as eggs and meat, is positively regarded for preventing sarcopenia and enhancing muscle protein synthesis (MPS) (Beasley et al., 2013). Additionally, plant-based protein sources, such as soybeans, peas, corn, and potatoes, are also effective in stimulating MPS (Nichele et al., 2022).
Among amino acids, those that the body cannot synthesize, such as leucine, are important for muscle formation. Recent studies have shown that consuming sulfur-containing amino acids, such as methionine and cysteine, helps mitigate muscle atrophy caused by various factors (Ellwood, 2022; Papet, 2019). Notably, organic sulfur amino acid derivatives found in garlic (Gupta et al., 2020), such as S-allyl-L-cysteine (SAC) (Gupta et al., 2020) and S-methyl-L-cysteine (SMLC) (Chae et al., 2023), are valued for their efficacy in mitigating muscle atrophy and promoting muscle differentiation and regeneration.
In light of these findings, this study seeks to explore the potential of black soybeans as a key ingredient in senior-friendly foods, particularly for preventing or mitigating age-related sarcopenia. To this end, we focused on identifying the optimal conditions for maximizing the content of sulfur-containing amino acids and active ingredients in the preparation of a low molecular weight fermented composition of enzyme-treated black soybeans.
2. Materials and methods
For this study, we purchased black soybeans of the Saebaram variety from Sangju, Gyeongbuk, harvested in October 2022. To compare the content of active ingredients, we also acquired other soybean varieties: Bitnadu (Sangju,Gyeongbuk), Gyeongheukcheong (Gumi, Gyeongbuk), and Cheongja5 (Sangju, Gyeongbuk), all harvested in the same month. The carbohydrate-degrading enzyme, Spezyme FRED, was purchased from Genencor (Rochester, NY, USA). Additionally, Prozyme GA, an enzyme capable of degrading both carbohydrates and proteins, was obtained from Bision Co. (Seongnam, Korea).
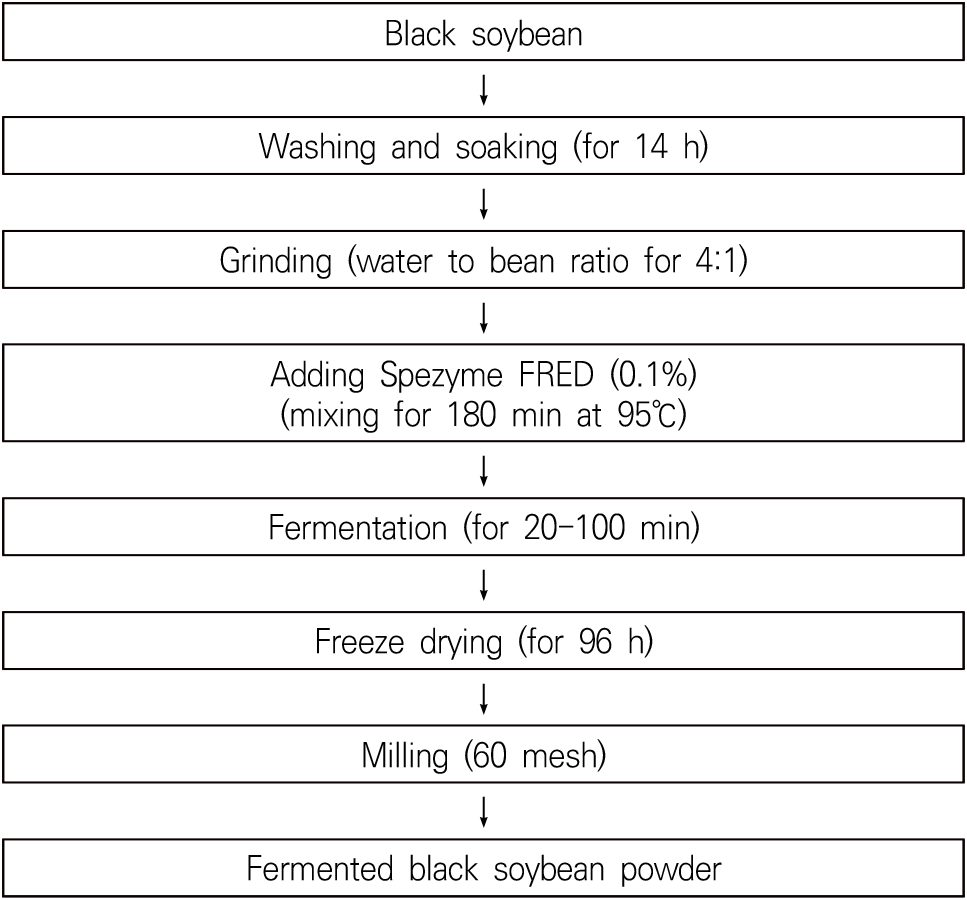
Fig. 1 illustrates the process of producing freeze-dried powder from fermented black soybeans. Initially, black soybeans of the Saebaram variety were washed and soaked for 14 h, followed by fine grinding. The resulting black soybean powder was blended with four times its weight in purified water and 0.1% (w/w) Spezyme FRED. This mixture was then steamed for 180 min at 95°C in a thermostatic water bath (JSMI-04T, JSResearch Inc., Gongju, Korea) for experimental purposes. To obtain the fermented black soybean products, fermentation was conducted under various conditions, with enzyme concentrations ranging from 0.1 to 0.5% and fermentation times varying from 20 to 100 min. These products were initially frozen at −40°C and subsequently dried for 96 h at 10°C in a freeze dryer (DRC-1000, FDU-2100, Eyela, Tokyo, Japan) until the moisture content reached 5.50±1.33%. Each freeze-dried black soybean product was ground, sieved through a 60-mesh standard sieve, and stored at room temperature for experimental use.
In the central composite design (CCD), the classified the independent variables (Xi) into five levels of enzyme concentration (X1) and fermentation time (X2), as outlined in Table 1. The study focused on several dependent variables (Yn): yield (Y1), amino nitrogen (Y2), β-glucan content (Y3), methionine content (Y4), and cystine content (Y5). We determined the central values and ranges for these independent variables based on preliminary experiments. Their mean values, obtained from triplicate measurements under 10 experimental conditions following the CCD, were utilized in regression analysis. We conducted response surface regression analysis using SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA). To visualize the features of these variables and their optimal extraction conditions, we created three-dimensional response surfaces with Minitab software (version 15, Minitab Inc., State College, PA, USA).
| Fermentation conditions | Coded level1) | |||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| X1 | Enzyme concentration (%) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 |
| X2 | Fermentation time (min) | 20 | 40 | 60 | 80 | 100 |
The yield of the fermented black soybean product was calculated using the following formula, which takes into account the weight of the original black soybeans and the weight of the freeze-dried powder:
The amino nitrogen content was determined using the formol titration method, as described by Im et al. (2002). This process involved preparing two key solutions. Solution A was prepared as follows: 5 g of the sample was dissolved in 100 mL of distilled water and stirred for 1 minute; the solution was then centrifuged at 4,000 × g for 15 min using a LaboGene 1248 centrifuge (Gyrozen, Daejeon, Korea). The supernatant was collected, mixed with 0.1 N NaOH, and titrated using a pH meter Orion Star A111 (Thermo Scientific Co., Waltham, MA, USA) until a pH of 8.4 was reached. Solution B was prepared by titrating a neutral formalin solution (35%) with 0.1 N NaOH until a pH of 8.4 was achieved. For determining the amino nitrogen content, 20 mL each of solutions A and B were combined. This mixture was then titrated with 0.1 N NaOH, and the amino nitrogen content was calculated as a percentage of milligrams.
The β-glucan content was analyzed using the Megazyme Kit (Mushroom and Yeast β-glucan Assay Procedure K-YBGL, Megazyme Ltd., Bray, Ireland), following these steps: 10 mg of the sample was mixed with 2 mL of 12 M sulfuric acid, and the mixture was left to react in a cold-water bath for 2 h. The solution was then diluted with 10 mL of distilled water and further reacted at 100°C for 2 h. After cooling, it was treated with 6 mL of 10 M potassium hydroxide. From this mixture, a 100 mL solution was prepared using 200 mM sodium acetate buffer (pH 5.0) and centrifuged at 1,500 × g for 10 min. The supernatant was collected, and 0.1 mL of it was mixed with 0.1 mL of exo-1,3-β-glucanase (20 U/mL) + β-glucosidase (4 U/mL). This mixture was left to react in a thermostatic water bath at 40°C for 1 h. Then, 3 mL of glucose oxidase/peroxidase mixture (GOPOD) was added and allowed to react at 40°C for 20 min. The absorbance of the reaction product was measured at 510 nm using a spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) to determine the total glucan content.
Additionally, a 20 mg sample was mixed with 2 mL of 20 M potassium hydroxide and the mixture was allowed to react in a cold-water bath for 20 min. To the reaction product, 8 mL of 1.2 M sodium acetate buffer (pH 3.8) and 0.2 mL of amyloglucosidase (1,630 U/mL) + invertase (500 U/mL) were added. This mixture was then left to react further at 40°C for 30 min. Subsequently, the solution was centrifuged at 1,500 × g for 10 min. Then 0.1 mL of the supernatant was mixed with 3 mL of GOPOD and left to react at 40°C for 20 min. The absorbance at 510 nm was measured to determine the α-glucan content. The β-glucan content (%) was then calculated by subtracting the α-glucan content from total glucan content.
One gram of the sample was mixed with 10 mL of 6 N HCl and hydrolyzed at 110°C for 22 h. The hydrolyzed mixture was then filtered using filter paper. For derivatization, 10 μL of the hydrolyzed sample was taken and mixed with 70 μL of borate buffer and 20 μL of AccQ-Fluor reagent. This mixture was stirred well for 10 s, left to react for 1 min, and then heated in a 55°C oven for 10 min. After cooling the reaction product to room temperature, it was used as the final test solution for analysis using HPLC (Alliance 2695, Waters, Milford, MA, USA). The analysis conditions were as follows: fluorescence detector with λex (250 nm) and λem (395 nm); column: AccQ Tag column (3.9×150 mm, Waters); flow rate: 1.0 mL/min; injection volume: 10 μL; mobile phase A: aqueous buffer (AccQ Tag Eluent A: DW=1:10); and mobile phase B: 60% acetonitrile. The standard solution for calibration was prepared by dissolving methionine and cystine in distilled water and diluting it stepwise. The content of each amino acid (expressed in mg%) was determined from the standard calibration curve prepared using the standard solution.
The optimal range of fermentation conditions was predicted by superimposing the response surfaces of each dependent variable to maximize their values. Moreover, a random point within the predicted range was selected and substituted into the regression equation. The predicted values were then compared with the experimental values obtained under the same conditions. The significance of the results was verified using the t-test in the SAS program (version 9.4, SAS Institute Inc.), with the significance level set at p<0.05.
Under the predicted optimal fermentation conditions, comparisons were made among the black soybean varieties in terms of yield (%), amino nitrogen content (mg%), β-glucan content (%), and the content of methionine and cystine (mg%).
3. Results and discussion
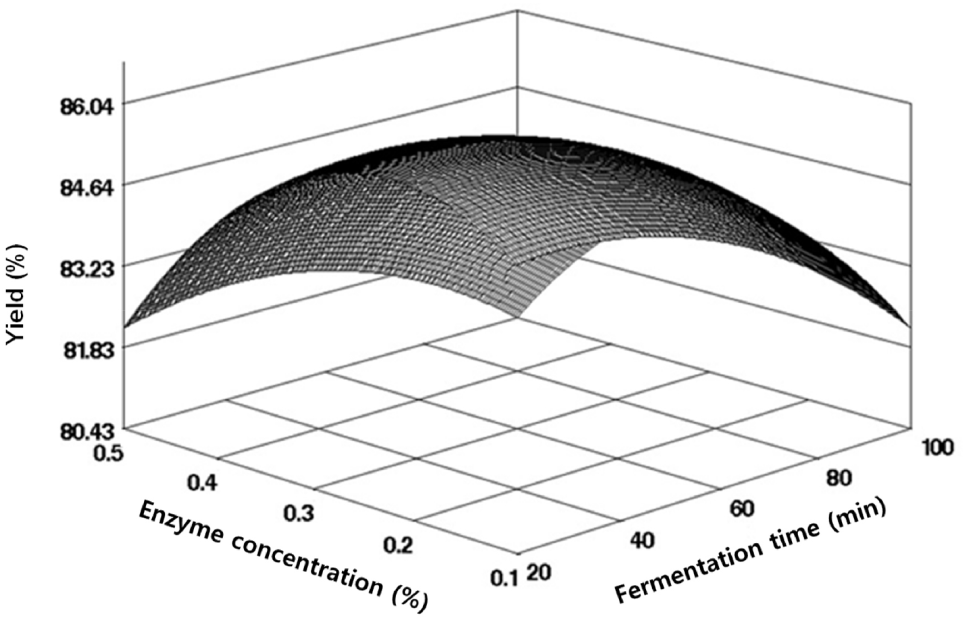
To produce freeze-dried powder from black soybean fermentation products enriched in sulfur amino acids, we monitored the yield using CCD-based response surface methodology (RSM). The yield ranged between 82.23% and 86.17%, as detailed in Table 2. The regression equation derived from the response surface regression analysis had an R2 value of 0.9027, indicating high reliability at a 5% significance level, as outlined in Table 3. The RSM-predicted optimum point was identified at the maximum point (Fig. 2). The maximum yield of 86.04% was predicted at an enzyme concentration of 0.23% and a reaction time of 32.44 min. The effect of enzyme concentration on yield during the fermentation of black soybeans was notably significant, as indicated in Table 4.
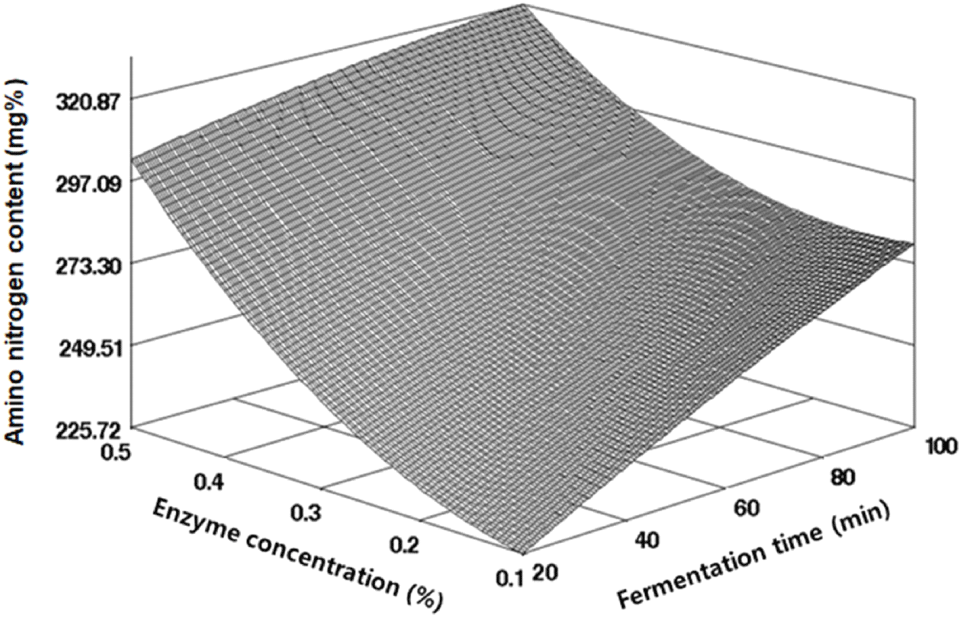
The amino nitrogen content, an indicator of protein breakdown into amino acids by protease, serves as a quality measure in fermented foods. Therefore, we monitored the amino nitrogen content during the fermentation of black soybeans to assess fermentation progression. Under the fermentation conditions set by the CCD, the amino nitrogen content in black soybeans ranged from 240.54 to 319.16 mg%, as indicated in Table 2, showing variations according to enzyme concentration. The regression equation from the response surface regression analysis, presented in Table 3, had an R2 value of 0.9150, signifying statistical significance at a 5% level. The optimal point for amino nitrogen content, predicted by RSM, was a saddle point (Fig. 3). The maximum value of 314.54 mg% occurred under conditions of 0.50% enzyme concentration and 65.87 min of fermentation time, while the minimum value of 237.48 mg% was observed under conditions of 0.18% enzyme concentration and 28.21 min of fermentation time, as detailed in Table 4. Kim et al. (2011) observed a similar range of amino nitrogen content (262.41-369.39 mg%) in the early stages of soybean paste fermentation from sprouted soybeans and black soybeans. Conversely, Song and Jung (2006) reported higher amino nitrogen contents in lactic acid-fermented soybeans and cheonggukjang (fast-fermented soybean paste), at 517.2 and 468.1 mg% respectively. However, these differences may be due to the use of different soybean varieties, fermentation methods, and strains.
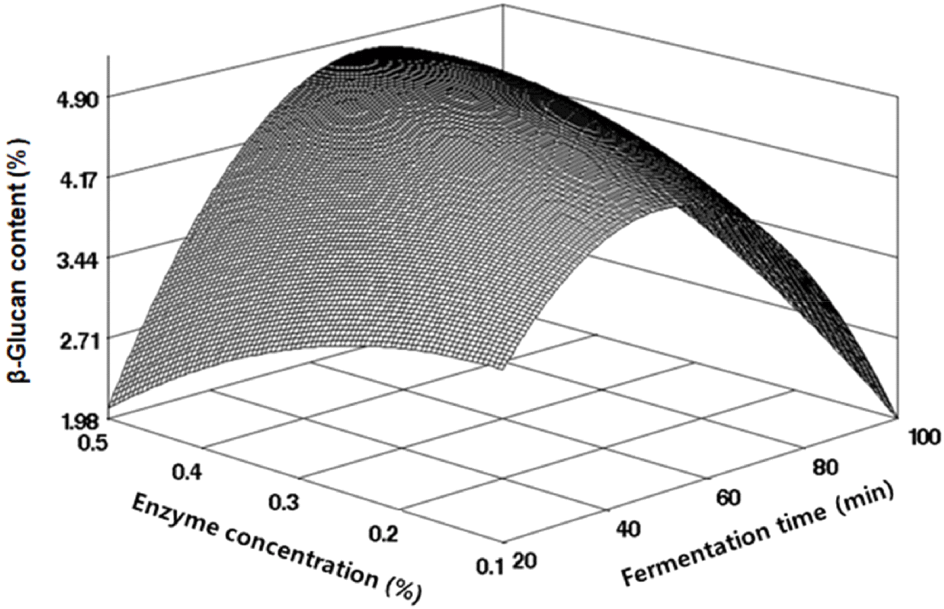
The analysis of β-glucan content in freeze-dried fermented black soybean powder, produced under various fermentation conditions, indicated a range from 3.05 to 5.07%, as outlined in Table 2. Regression analysis of these results derived an R2 value of 0.8439 from the regression equation, confirming its significance within a 10% level, as detailed in Table 3. The RSM-predicted optimal point, identified as the maximum point (Fig. 4), indicated the highest β-glucan content of 4.90% at an enzyme concentration of 0.36% and fermentation time of 64.27 min. The change in β-glucan content during black soybean fermentation was more significantly influenced by fermentation time than by enzyme concentration, as indicated in Table 4. β-glucan, known for its abundance in mushrooms, yeast, and cereals, has been reported to positively impact immunity and anti-cancer activity, and to improve blood circulation (Hirokazu et al., 1989; Lee and Bang, 2001; Misuno, 1990). The maximum β-Glucan content predicted in this study for fermented black soybean products was comparable to the range (3.76-4.60%) found by Lee et al. (2017) in various uncut oat varieties cultivated in Korea, as well as to the 5.55% β-glucan content in Cordyceps militaris extracted through enzymatic hydrolysis at 40°C for 4 h, as reported by Gwon et al. (2022). Lee and Lee (2009) also reported a similar range of β-glucan content (1.06-12.60%) in soybeans cultured with various mushroom mycelia.
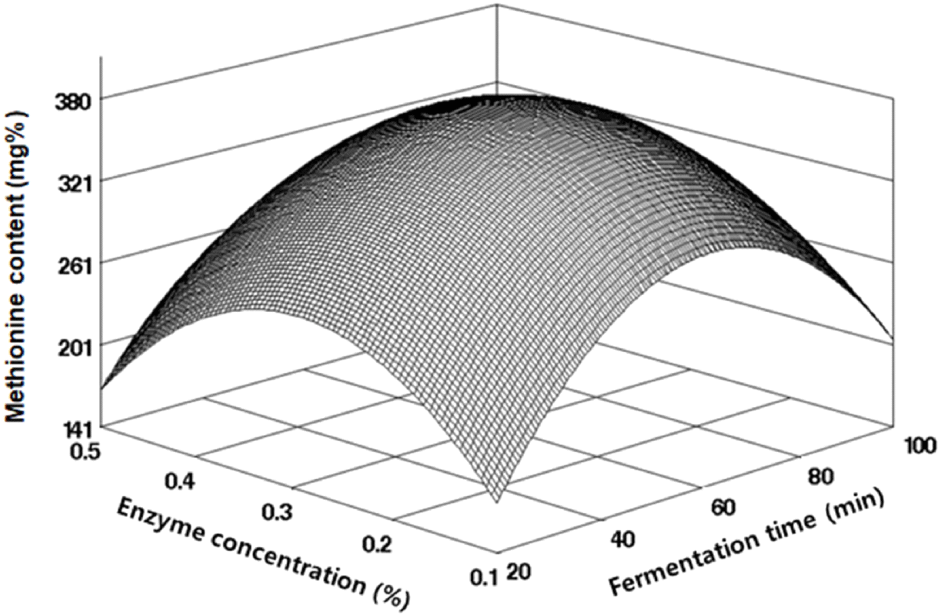
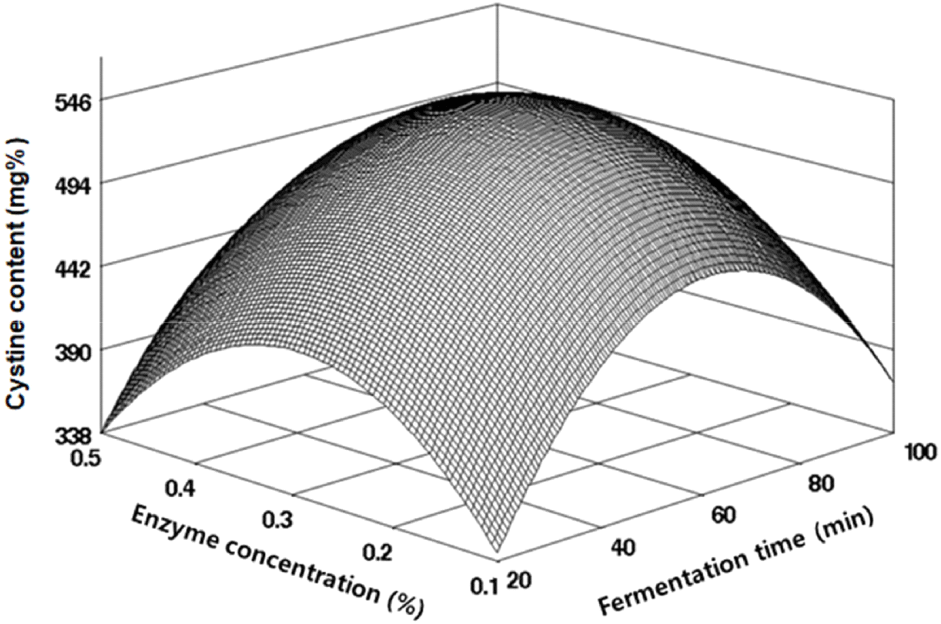
Methionine and cystine, sulfur-containing amino acids, are recognized for their roles in improving muscle atrophy and constituting skin and hair; notably, methionine is identified as a limiting amino acid in legumes. To produce black soybean fermentation products rich in sulfur amino acids, we analyzed methionine and cystine content under various fermentation conditions, as shown in Table 2. The methionine content ranged from 270.83 to 401.37 mg%, and cystine ranged from 430.28 to 570.22 mg%. Regression analysis yielded R2 values of 0.9145 for methionine and 0.8457 for cystine, confirming their significance within 5% and 10% levels, respectively, as indicated in Table 3. Their RSM-predicted optimal points coincided with the maximum values (Figs. 5 and 6). The highest predicted value for methionine was 380.23 mg% at an enzyme concentration of 0.28% and fermentation time of 60.36 min, while for cystine, it was 545.86 mg% at an enzyme concentration of 0.29% and fermentation time of 61.34 min, as outlined in Table 4. Im et al. (2016) reported different ranges for various soybean varieties, with methionine ranging from 520 to 540 mg% and cystine from 640 to 660 mg%, suggesting that factors such as soybean variety, growth environment, and processing methods can influence these levels.
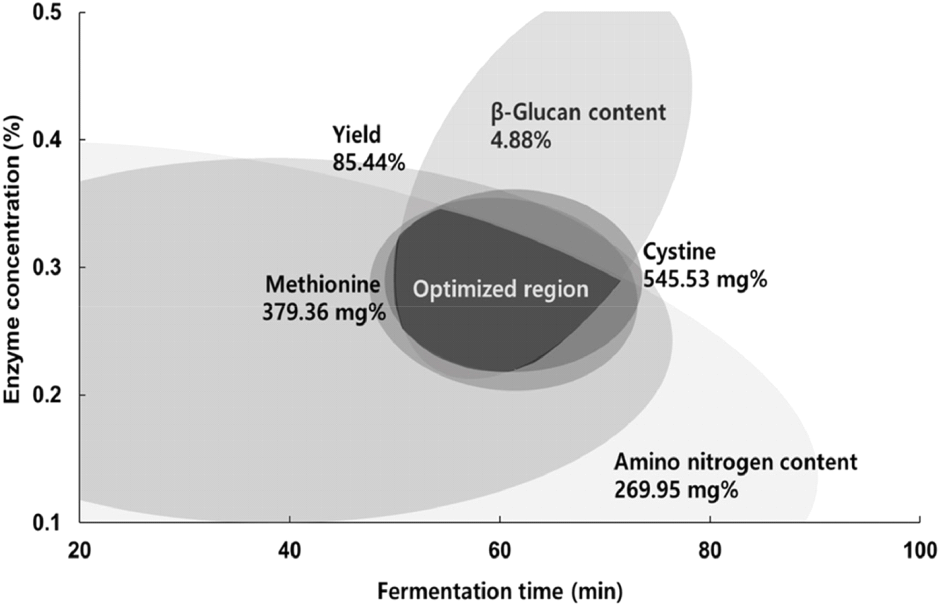
To establish conditions for fermenting black soybeans rich in sulfur amino acids, we superimposed response surfaces for each variable as depicted in Fig. 6. This superimposition of response surfaces for maximum values, as derived from individual items’ optimal fermentation conditions, resulted in a complete or partial overlap for the response surface plots of five variables, including yield, amino nitrogen content, and β-glucan content. The range of each independent variable within the overlapping areas was defined as the optimal extraction condition range. This range, represented by the darker area in Fig. 7, was predicted to be between 0.28 to 0.32% for enzyme concentration and 58.0 to 62.0 min for fermentation time, as shown in Table 5. We verified the accuracy of the regression model by conducting experiments under these optimal conditions (0.30% enzyme concentration and 60 min fermentation time) and comparing the predicted values with the actual results, detailed in Table 6. The outcomes revealed comparable values for yield (85.58±0.33%) and amino nitrogen content (270.02±0.81 mg%), as well as for methionine and cystine (378.98±9.45 and 561.52±9.98 mg%, respectively), although the β-glucan content was slightly lower at 4.80±0.16%. The regression model demonstrated an appropriate fit, yet significant differences were observed between the predicted and actual values, as indicated in Table 6.
| Fermentation conditions | Range of predicted condition1) |
|---|---|
| Enzyme concentration (%) | 0.28-0.32 |
| Fermentation time (min) | 58.0-62.0 |
The analysis of experimental results for active ingredients in black soybean fermentation under optimal conditions for each variety revealed significant differences. The yields of Gyeongheukcheong (87.55%) and Bitnadu (86.89%) were significantly higher (p<0.05) than those of Saebaram (85.58%) and Cheongja 5 (83.90%), as shown in Table 7. The amino nitrogen content was significantly higher in Saebaram (270.02 mg%) and Gyeongheukcheong (269.08 mg%) compared to Bitnadu (244.28 mg%) and Cheongja 5 (224.63 mg%). The β-glucan content was significantly higher (p<0.05) in Saebaram (4.80%) and Gyeongheukcheong (4.61%) than in Cheongja 5 (3.94%) and Bitnadu (3.29%). Methionine content varied by variety, ranging from 350.29 to 412.38 mg%, with the highest value in Gyeongheukcheong (483.38 m%). Cystine content ranged from 455.7 to 598.65 mg%, with the highest value in Cheongja 5 (598.65 mg%), showing statistically significant differences (p<0.05). The total content of sulfur-containing amino acids (methionine and cystine) exhibited inter-variety differences, in the order of Bitnadu (993.62 mg%), Saebaram (940.50 mg%), and Gyeongheukcheong (939.09 mg%).
4. Conclusions
This study monitored the content of active ingredients in black soybean fermentation products rich in sulfur amino acids using RSM, we optimized fermentation conditions for freeze-dried powder of fermented black soybeans. The response surface model, based on the CCD, identified enzyme concentration and fermentation time as key independent variables (Xi). The yield, amino nitrogen content, β-glucan content, methionine content, and cystine content were set as five dependent variables (Yn). Regression analysis of 10 conditions predicted optimal conditions for each variable, which were subsequently validated experimentally. As a result, the optimal fermentation conditions for black soybean powder high in sulfur amino acids were estimated to be between 0.28 to 0.32% enzyme concentration and 58.0 to 62.0 min of fermentation time. The sulfur amino acid content varied by soybean variety under these optimal enzymatic treatment conditions, ranging from 818.16 to 922.62 mg%. The findings of this study provide foundational data for developing fermentation products using black soybeans as functional foods, especially targeted at the nutrition of older adults.