1. Introduction
Lemons (Citrus limon [L.] Burmese. f) are a popular citrus fruit consumed worldwide; their global production is the third highest of all citrus fruits, after mandarins and oranges (Zhang et al., 2018). Lemons are native to Southeast Asia and grow primarily in northeastern India, Myanmar, China, California, Italy, Spain, and Australia (Al-Jabri et al., 2014). They have a tart taste and a vibrant yellow color and consist of flavedo, albedo, and pulp. Additionally, they contain various physiologically active substances, such as carotenoids and flavonoids.
Among all fruits, citrus contains the most diverse types of carotenoids, and their type and content vary depending on the cultivar and growing environment. The yellow color in the flavedo and juice sacs of mature lemons is due to the presence of carotenoids, particularly β-cryptoxanthin (Kato et al., 2004). Lemons also have a high organic acid content, including citric acid, malic acid, ascorbic acid (vitamin C), and tartaric acid (Nour et al., 2010). Hydrocarbons, alcohols, esters, and aldehydes are representative volatile components of lemons and are responsible for their characteristic aromas (Espina et al., 2011). Citrus fruits are rich in flavonoids, including hesperidin, hesperetin, naringin, naringenin, quercetin, rutin, and tangeretin (Sun et al., 2013). These flavonoids are also present in lemons and possess some health-promoting effects, such as free-radical scavenging and antibacterial, anti-inflammatory, and antioxidant properties (Addi et al., 2022; Mahmoud et al., 2019).
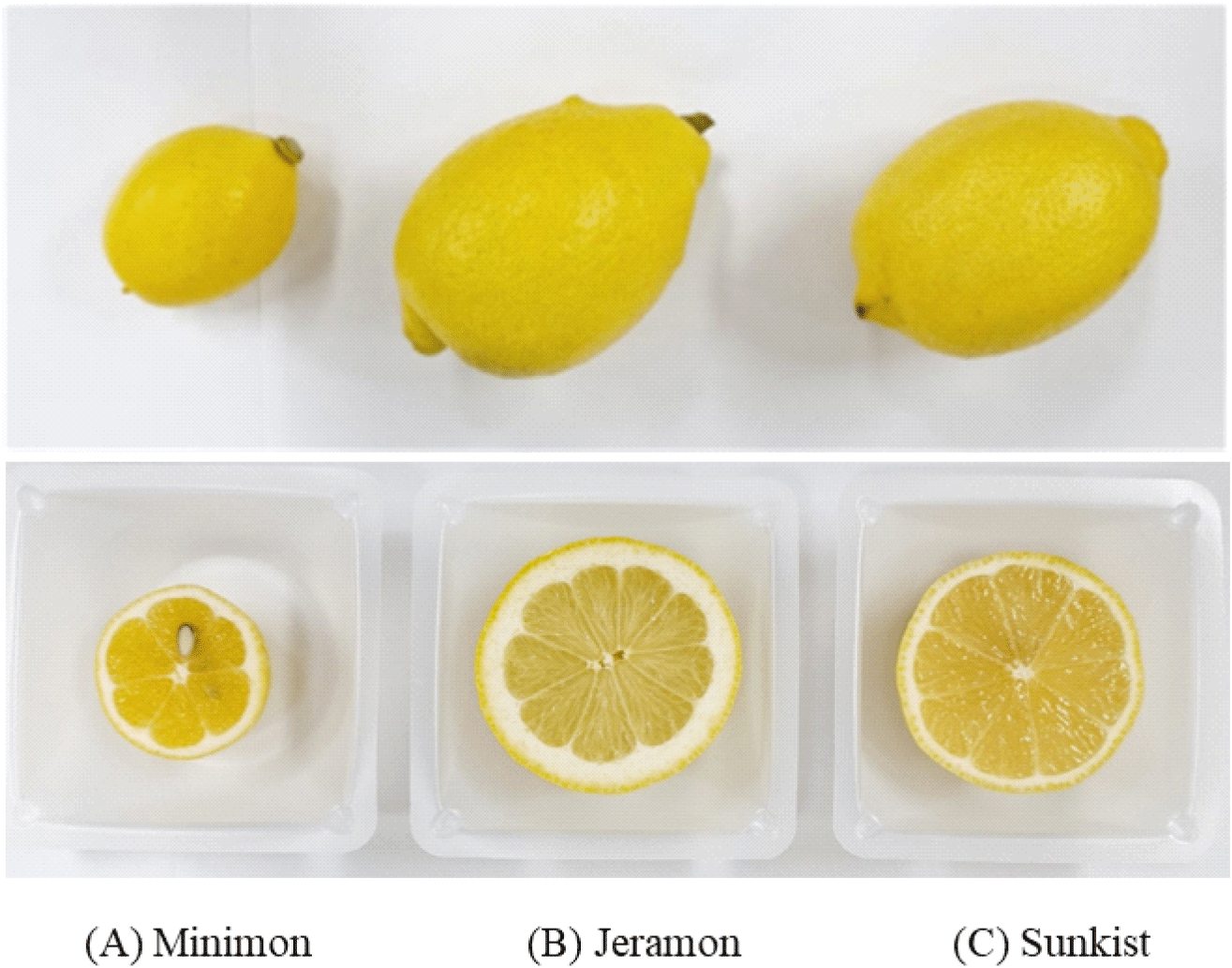
Owing to the numerous benefits of lemons mentioned above, their consumption is increasing not only in the beverage and baking industries but also in households. Ninety percent of lemons distributed in Korea are imported, mainly from the United States and Chile. These imported lemons are coated with wax or treated with preservatives for long-term transport and storage; therefore, there is a high demand for fresh domestic lemons. Jeramon (registration No. 9060) is a new lemon cultivar and nucellar mutant that was developed from C. limon ‘Frost Lisbon’ and C. limon ‘Mayor;’ it was first developed in Korea (Woo et al., 2020) to cope with the increasing imports. The Jeramon variety was specifically bred and selected to grow in Korea, which has a relatively low temperature and is cultivated in Korea’s southernmost Jeju Island. Jeramons weigh approximately 140 g, which is relatively large compared to normal lemons (130 g). The peel is approximately 5 mm thick, its juice contains 11° Brix, and its acid content is 8.5%, approximately 1% higher than common lemons (Agricultural Technology, 2022).
Minimon (registration No. 9059) is an ornamental lemon variety bred by seedling (C. limon ‘Mayor’) in Korea (Woo et al., 2020). The Minimon variety was also developed to meet the demand for fresh domestic lemons and to diversify varieties. Its flowers bloom three times a year, and the fruit is small; therefore, Minimon is suitable for raising at home. It is also available as a raw fruit. Minimons are very small, weighing approximately 40 g. The fruit is round, and its skin and pulp are light yellow (Ministry of Culture, Sports and Tourism, 2019).
Lemon varieties such as Minimon and Jeramon have been developed in Korea, but only their appearance (size and shape) and basic characteristics (sweetness and acid content) have been analyzed; no research has been conducted on their color, taste, and aroma, which are the essential characteristics. Therefore, this study aimed to analyze the major quality characteristics, such as the pigments, volatile components, and antioxidant properties, of the newly developed lemon varieties in Korea and compare them with the globally popular common lemon.
2. Materials and methods
Citric acid, malic acid, tartaric acid, succinic acid, oxalic acid, ascorbic acid, and potassium phosphate were acquired from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile was obtained from J.T. Baker (Phillipsburg, NJ, USA). β-Cryptoxanthin was purchased from Carotenature (Lupsingen, Switzerland).
Minimon and Jeramon harvested in October 2022 were kindly provided by the Agricultural Technology Center R&D Division (Goyang, Korea). The Sunkist lemon, the most consumed lemon variety in Korea, was purchased at the Hyundai department store (Mia store, Seoul, Korea). Whole lemons were washed and cut into pieces before being lyophilized with a freeze dryer (FD8512, IlShinBioBase Co., Yangju, Korea) at for three days, and stored at −80°C for analysis.
The weights of seven randomly selected samples from each variety were determined (GH-200, A&D, Tokyo, Japan) and average weights were considered for further analysis. After cutting three samples of each variety in half, the juice was collected using a juice extractor. The mixture of the juice was centrifuged at 3,600 rpm×15 min at 4°C in a centrifuge (Sorvall X1R Pro, Thermo Fisher Scientific, Waltham, MA, USA). The sugar content and pH of the supernatant samples centrifuged were analyzed. The sugar contents were measured using a refractometer (PAL-1, Atago, Tokyo, Japan). The pH was measured by a pH meter (Orion Star™ A211, Thermo Fisher Scientific).
β-Cryptoxanthin was analyzed using the method of Hwang et al. (2015). A Dionex ASE 350 system (Dionex Co., Sunnyvale, CA, USA) was used to perform accelerated solvent extraction (ASE). A cellulose filter was inserted into the 22 mL extraction cell, followed by the insertion of an ASE-non-stick thimble (Whatman, Schleicher & Schuell Bioscience, Dassel, Germany). Subsequently, 1 g of powdered sample was mixed with diatomaceous earth (ASE Prep Dionex), placed in the extraction cell, and extracted with acetone as the solvent.
The extraction conditions were as follows: static time: 3 min; static cycles: 3; nitrogen purge: 60 s; pressure: 1,500 psi; and temperature: 100°C. The extracted sample was concentrated using a nitrogen evaporator in a water bath at 60°C and used for saponification. Sample saponification was performed by adding 3 mL of acetone to the concentrated sample, re-dissolving it, and mixing it with 3 mL of methanol and 1 mL of 30% potassium hydroxide/methanol (v/v); the sample was then left in the dark for two and a half hours. The saponified sample was extracted step by step with diethyl ether, distilled water, 10% sodium chloride, and 2% sodium sulfate before being separated into layers to ensure that only pure carotenoids were transferred to the diethyl ether layer. After solvent fractionation, the diethyl ether layer was concentrated, dissolved in 2 mL of acetone, and filtered with a 0.2 μm syringe filter for further analysis.
β-Cryptoxanthin was analyzed using ultra-performance liquid chromatography (UPLC; Acquity UPLC H-Class, Waters, Milford, MA, USA) with an HSS T3 column (2.1 mm×100 mm, 1.8 μm, Waters), acetonitrile/methanol/methylene chloride (65:25:10, v/v/v) as mobile phase A, and tertiary distilled water as mobile phase B. The gradient conditions of the mobile solvent were 0-6.5 min, 30% B; 6.5-7 min, 30-25% B; 7-11 min, 25% B; 11-11.5 min, 25-30% B; 17 min, 30% B; 17-17.5 min, 30-0% B; 27.5 min, 0% B; 27.5-28 min, 0-30% B; 30 min, 30% B. Flow rate was 0.5 mL/min, oven temperature was 35°C, injection volume was 1.0 μL, and the ultraviolet detector wavelength was 450 nm.
The β-cryptoxanthin standard was dissolved in dimethyl sulfoxide to prepare a stock solution of 2 mg/mL, which was then diluted in 5 steps to a concentration in the range of 1-100 μg/mL. A calibration curve was obtained from the peak area at each concentration, and linearity and correlation (R2) were confirmed for carotenoid quantification.
Lyophilized lemon powder (100 mg) was mixed with distilled water (10 mL), vortexed for 1 min, and extracted at room temperature for 60 min. This mixture was centrifuged at 2,000 ×g for 10 min at 20°C. The supernatant was then filtered using a 0.45-μm syringe filter and used as the sample for ascorbic acid analysis. The same method was followed to prepare samples for organic acid analysis (section 2.6).
Ascorbic acid was measured using an e2695 HPLC system (Waters) equipped with a YMC PACK ODS-AM column (4.6 mm×250 mm, 5 μm; YMC, Kyoto, Japan), using the method of Kim et al. (2022a) with some modifications. The mobile phase was 50 mM potassium phosphate in acetonitrile (60:40, v/v). The flow rate was 0.5 mL/min, the column oven was set at 40°C, injection volume was 10 μL, and the detection wavelength was 264 nm.
Samples for organic and ascorbic acid analyses were prepared using the same method (described in section 2.5). The organic acid analysis parameters and organic acid content were analyzed using an ACQUITY UPLC H-Class system, coupled with a tunable ultraviolet detector and a Xevo triple quadrupole mass spectrometer (Waters), that was equipped with an ACQUITY HSS T3 column (Waters). Mobile phases were 0.1% formic acid in distilled water (A) and 0.1% formic acid in acetonitrile (B). Gradient elution was set as follows: 0 min, 2% B; 0-3.5 min, 10% B; 3.5-3.6 min, 2% B; 3.6-5.0 min, 2% B. The flow rate was 0.3 mL/min, and the injection volume was 5 μL. The column oven was maintained at 45°C. Organic acids were ionized using electrospray ionization in negative ion mode. The mass-to-charge ratios (m/z) of precursor and product ions were detected using multiple reaction monitoring modes. The capillary voltage was set at 2.8 kV and cone voltages at 50 V. Desolvation and source temperatures were 350 and 150°C, respectively. The flow rates of the desolvation and cone gases were 800 and 50 L/h, respectively. The collision energy was 40 V. Data were acquired using MassLynx software V.4.1 (Waters).
After adding 10 mL of 80% methanol to 1 g of the powdered sample and extracting it with an ultrasonic sonicator (8510, Branson, Danbury, CT, USA) for 1 h, the extract was centrifuged at 1,800 ×g for 10 min at 20°C. The supernatant was filtered using filter paper (Advantec NO.2, Tokyo, Japan) and concentrated using a nitrogen evaporator in a water bath at 60°C. The concentrated sample was then lyophilized.
Lyophilized samples were weighed and re-dissolved in 80% methanol, filtered using a 0.45 μm syringe filter, and used as a stock solution (200 mg/mL).
ABTS radical scavenging activity was measured by the modified method of Re et al. (1999). Ten milliliters of 7 mM ABTS and 2.45 mM potassium sulfate were mixed and kept in the dark for 24 h at room temperature before use. The ABTS•+ solution was diluted with ethanol to have an absorbance of 0.7±0.02 at 734 nm, which was measured using a spectrophotometer (OPTIZEN α, Mecasys, Daejeon, Korea). Subsequently, 990 μL of diluted ABTS•+ solution and 10 μL of the sample were mixed and kept in the dark at room temperature for 5 min. Absorbance was measured at 734 nm. The relative ABTS•+ radical scavenging activity was calculated using the following equation:
DPPH radical scavenging activity was measured using the modified methods of Brand-Williams et al. (1995) and Sarpras et al. (2019). Sample extract (100 μL) and 100 μM DPPH solution (900 μL) were mixed and kept in the dark at 24°C for 30 min. Absorbance was measured at 517 nm using a spectrophotometer. DPPH radical scavenging activity was obtained by the following equation:
Volatile compounds were extracted from the lemon samples using headspace-solid phase microextraction (HS-SPME) and separated using gas chromatography-mass spectrometry (GC-MS) by the method of Zhang et al. (2007) with minor modification. One gram of each sample was inserted in a 20-mL vial capped with silicone septa (Sigma Chemical Co., St. Louis, MO, USA). SPME fiber (85 μm DVB/CAR/PDMS, Supelco, Bellefonte, PA, USA) was placed into the inlet of the GC injector at 250°C for 30 min. The fiber was exposed to the headspace of the sample, where extraction was allowed to occur for 20 min with continuous agitation at 40°C. The fiber was subsequently desorbed in the GC injector for 5 min.
Volatile compounds were analyzed using an Agilent 8890 GC (Agilent Technologies Inc., Santa Clara, CA, USA) connected with an Agilent 5977B mass spectrometer system. The column used was an HP-5MS (cross-linked 5%-phenyl-methylpolysiloxane) capillary column with dimensions of 30 m×0.25 mm i.d×0.25 μm film thickness (Agilent Technologies Inc.). The carrier gas was helium at a flow rate of 1.0 mL/min. Desorption of analytes from the SPME fiber was carried out at 250°C for 5 min with a splitless mode. The initial oven temperature was set at 50°C and held for 3 min, followed by a gradual increase to 150°C at a rate of 3°C/min, and finally increased to 240°C at a rate of 20°C/min and kept for another 10 min. The mass spectra were obtained in an electron ionization mode at 70 eV, and the range of mass-to-charge ratio (m/z) was from 35 to 550 atomic mass units. The identification of volatile compounds was performed by comparison of retention index and times with those obtained for authentic standards or those available in literature, or with mass spectra in the NIST 17 mass spectral library.
Data were subjected to a one-way analysis of variance, followed by a post-hoc test using Duncan’s multiple range test to identify the significance of differences among means at p<0.05, using IBM SPSS Statistics for Windows (Version 25.0, Armonk, NY, USA). All data are expressed as mean±standard deviation (n=3).
3. Results and discussion
The weight of the Minimon variety was 57.53 g, which is approximately 30% of the 140.17 g of Jeramon, and 143.95 g of Sunkist (Table 1). A cross-section of lemon samples (Fig. 1) revealed that Jeramon had a thicker peel than Sunkist, whereas Minimon had a thinner peel and more seeds.
| Weight (g) | Sugar content (°Brix) | pH | |
|---|---|---|---|
| Minimon | 57.53±7.71b1) | 9.37±0.06b | 2.38±0.04a |
| Jeramon | 140.17±13.33a | 9.57±0.06a | 2.25±0.03b |
| Sunkist | 143.95±8.14a | 6.60±0.0c | 2.32±0.03a |
The sugar contents of Minimon (9.37±0.06 g) and Jeramon (9.57±0.06 g) were significantly higher than that of Sunkist (6.60±0.00 g). Minimon and Jeramon, which contain a higher sugar content than existing lemons, are expected to increase the purchasing power of consumers looking for sweeter fruits. In addition, the pH of Jeramon (2.25±0.03) was significantly lower than that of Minimon (2.38 ±0.04) and Sunkist (2.32±0.03), indicating that Jeramon had a higher acid content.
Jeramon weighs approximately 10 g more, contains approximately 10% higher sugar content, and contains approximately 1% higher acid content than Lisbon, the original variety (Agricultural Technology, 2022). Jeramon is expected to compete with foreign lemon varieties because lemons with higher acidity are considered better. Additionally, Minimon is much smaller in size (130 g) but exhibits a higher sugar content and yellower peel than the original variety (Ministry of Culture, Sports and Tourism, 2019).
Out of the 10 different carotenoids tested during this study, only β-cryptoxanthin was detected in all three samples (Table 2). The β-cryptoxanthin content was the highest in Minimon (0.21±0.02 mg/100 g dry basis), followed by Jeramon (0.10±0.00 mg/100 g dry basis) and Sunkist (0.09±0.00 mg/100 g dry basis). From this, it can be observed that the new cultivar, Minimon, contains twice as much β-cryptoxanthin as Sunkist, whereas Jeramon contains a similar level to that of Sunkist; this confirms that these new lemon varieties are a rich source of β-cryptoxanthin.
| β-Cryptoxanthin (mg/100 g dry basis) | L-Ascorbic acid (mg/g dry basis) | |
|---|---|---|
| Minimon | 0.21±0.02a1) | 9.00±0.20b |
| Jeramon | 0.10±0.00b | 10.00±0.20a |
| Sunkist | 0.09±0.00b | 2.80±0.10c |
A typical characteristic of lemons is their yellow color; however, their carotenoid content is very low. Previous studies have reported a decrease in colored carotenoid expression in the flavedo and pulp of lemons during the transition from green to yellow, and a less complex carotenoid component in maturity compared to other citrus fruits (Alquézar et al., 2009; Kato et al., 2004; Matsumoto et al., 2007; Yokoyama and White, 1967).
β-Cryptoxanthin is a typical carotenoid and a natural compound derived from citrus. It possesses various biological activities, including antioxidant and anticancer effects, as well as provitamin A activity (Jiao et al., 2019). β-Cryptoxanthin is the most abundant yellow-colored carotenoid in the pulp of lemon varieties such as Meyer and Eureka Fr (Alquézar et al., 2009), which is consistent with our results.
L-Ascorbic acid is an important nutritional ingredient in citrus fruits. In this study, all lemons showed high L-ascorbic acid content, as shown in Table 2. The L-ascorbic acid contents of Jeramon (10.00 ± 0.20 mg/g dry basis) and Minimon (9.00 ±0.20 mg/g dry basis) were approximately three times higher than that of Sunkist (2.80±0.10 mg/g dry basis). L-ascorbic acid is generally considered to be the main antioxidant component in citrus fruits (Martí et al., 2009). Our data suggests that Minimon and Jeramon are good sources of L-ascorbic acid and possess enhanced antioxidant properties.
In a comparison study of citrus fruit juices, L-ascorbic acid levels in lemon juice ranged between 20 and 60 mg/100 mL of juice (Nagy, 1980). The L-ascorbic acid content of lemons varied with the variety. The L-ascorbic acid content in Fino juice (45.7-57.3 mg/100 mL) was higher than that in Eureka juice (32.4-36.4 mg/100 mL) and Lisbon juice (38.3-40.0 mg/100 mL) (Martí et al., 2009).
Organic acids are important for the overall flavor balance in foods and beverages and have various physiological effects (Quitmann et al., 2014). The organic acid analysis parameters and organic acid content of the different lemon varieties are shown in Tables 3 and 4, respectively. The major organic acids were citric and malic; the acidity of lemon juice is mainly attributed to these acids. If other acids were present, they would have been present in small amounts. The citric acid contents of Minimon (102.60 mg/g dry basis) and Jeramon (81.40 mg/g dry basis) were significantly lower than that of Sunkist (114.60 mg/g dry basis). Citric acid is the most abundant organic acid in lemons (Penniston et al., 2008) and contributes to their tart flavor. The low citric acid content of Minimons and Jeramons is associated with their low acidity, making these varieties good food ingredients. However, the malic acid contents of Minimon (30.60 mg/g dry basis) and Jeramon (27.20 mg/g dry basis) were significantly higher than that of Sunkist (7.10 mg/g dry basis). Malic acid is the main acidic component of numerous fruits, including apples, bananas, and plums, and is identified as a secondary acid substitute for citric acid in citrus fruits (Perera and Perera, 2019). The flavor of citrus fruits is strongly influenced by the levels of total soluble solids and organic acids (Frederick, 2009).
To measure the antioxidant activities of the three lemon extracts, DPPH and ABTS radical scavenging activities were analyzed. The results are shown in Fig. 2.
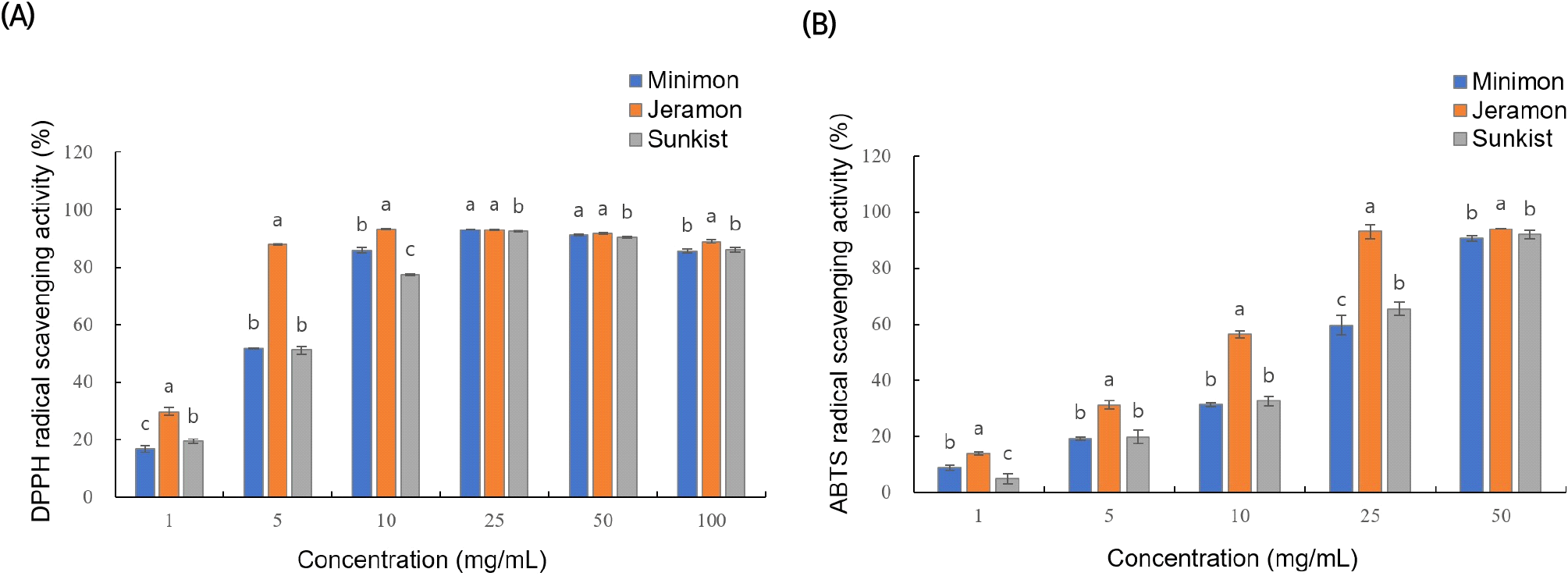
When the lemon extracts were used at concentrations of 1, 5, 10, 25, and 50 μg/mL, the DPPH radical scavenging activity increased in a concentration-dependent manner up to a concentration of 25 mg/mL (Fig. 2(A)). The DPPH radical scavenging activity of the Jeramon extract was much higher than that of the Sunkist or Minimon extracts at concentrations of 10 mg/mL or less. The DPPH radical scavenging activity of the Jeramon extract at a concentration of 10 mg/mL (93.2%) was similar to that of 100 μg/mL of L-ascorbic acid (93.5%) and 200 μg/mL of quercetin (92.3%).
A similar tendency was observed for ABTS radical scavenging activity. When the lemon extracts were added at concentrations of 1, 5, 10, 25, and 50 μg/mL, the ABTS radical scavenging activity showed a concentration-dependent tendency to increase up to a concentration of 50 μg/mL (Fig. 2(B)). The ABTS radical scavenging activity of the Jeramon extract was much higher than that of the Sunkist or Minimon extracts at a concentration of 25 mg/mL. The ABTS radical scavenging activity of the Jeramon extract at a concentration of 50 mg/mL (94.1%) is similar to that of 500 μg/mL of L-ascorbic acid (94.5%) and 500 μg/mL of quercetin (92.3%).
Upon antioxidant activity analysis by measuring DPPH and ABTS radical scavenging activities, the Jeramon extract showed the highest antioxidant activity, and the antioxidant activity of the Minimon extract was similar to that of Sunkist or slightly higher at higher concentrations. The differences between the DPPH and ABTS radical scavenging activities were examined because the DPPH radicals are free radicals, whereas the ABTS radicals are cationic (Wang et al., 1998). In addition, DPPH and ABTS radical scavenging assays showed different reaction kinetics and antioxidant potentials during reaction with phenolic compounds. When the reaction occurs, the ABTS radical must be induced initially, whereas DPPH is self-stabilized (Lemos et al., 2019).
It seems that Jeramon can also be used as a natural antioxidant. Reactive oxygen species have been implicated in the oxidative deterioration of foods and the pathogenesis of human diseases, such as diabetes, chronic inflammation, and certain types of cancer (Denkova-Kostova et al., 2020). Antioxidants prevent oxidative reactions by scavenging reactive oxygen species and can be natural or synthetic. Synthetic antioxidants are compounds with phenolic structures and varying degrees of alkyl substitutions, whereas natural antioxidants include vitamins, flavonoids, and phenolic compounds. There is interest in discovering naturally occurring antioxidants for usage in pharmaceutical or food applications to protect the human body from free radicals, retard lipid oxidation in food, and slow the progress of numerous chronic diseases (Prior, 2003). Most antioxidant compounds extracted from plant sources have been identified as free radicals and active oxygen scavengers (Ramarathnam et al., 1995).
Lemons are known for their abundance of flavor-enhancing components. The flavor of lemons is attributed to the combination of volatile (mainly alkenes, ketones, alcohols, aldehydes, and esters) and non-volatile (mainly soluble sugars, amino acids, and organic acids) flavor compounds (Jiang et al., 2022). In this study, volatile compounds were identified using linear retention indices and mass spectral data. Fig. 3. depicts the HS-SPME-GC-MS profiles of the samples. A comparison of the volatile compounds identified in the three lemon species is summarized in Table 5. As expected, the profile of the chromatograms revealed high similarity among the three types of lemon samples. In prior research, the most volatile components detected in citrus were esters, terpenoids, aldehydes, alcohols, and acids (Pichersky et al., 2006; Schwab et al., 2008).
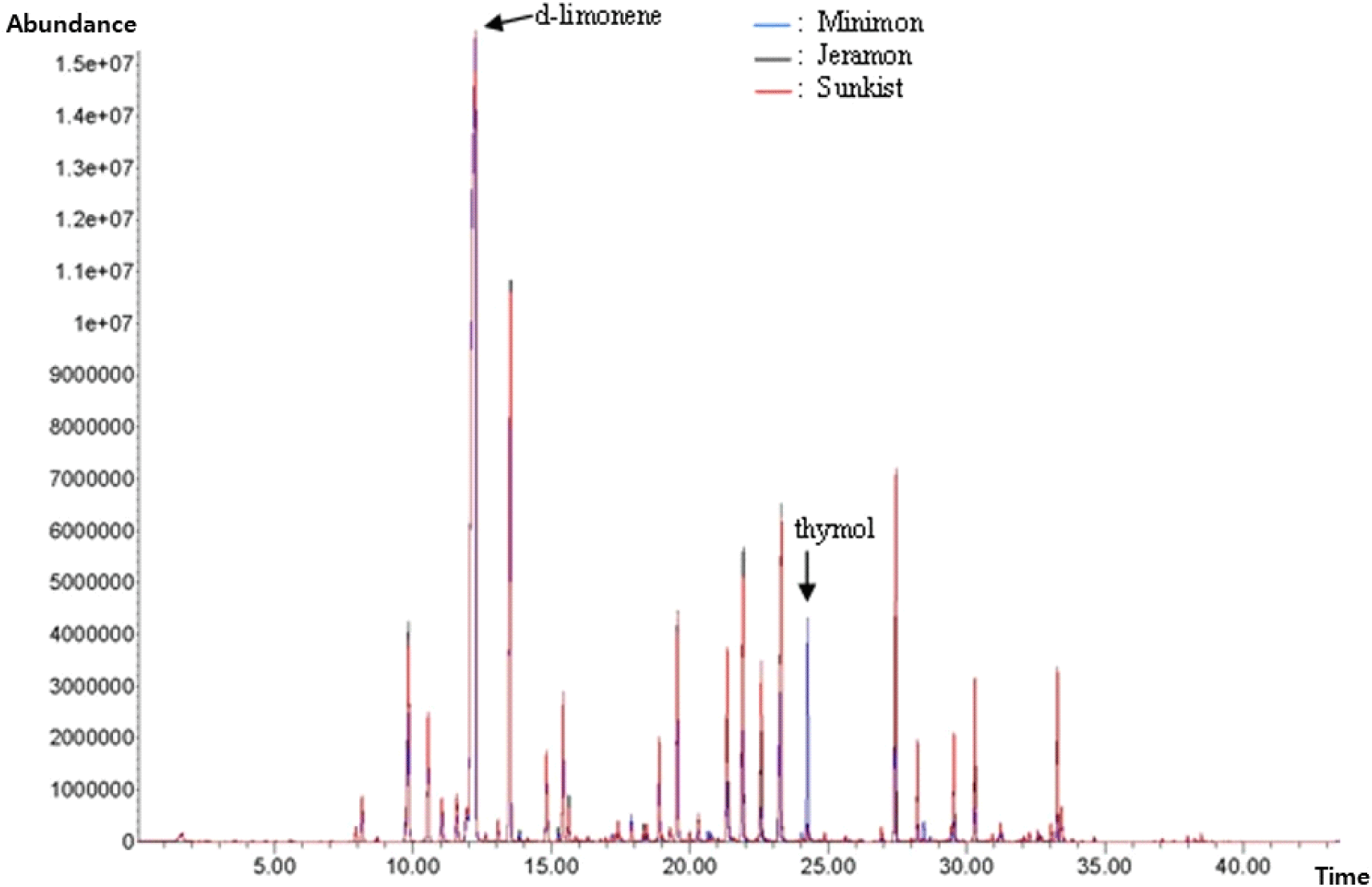
The volatile patterns of the three lemon samples were dominated by terpenoids, which are mostly found in citrus fruits and are essential for plant growth and development (Kim et al., 2022b). The most abundant terpenoids were d-limonene (33.69-45.42%), γ-terpinene (9.65-11.02%), β-pinene (3.56-4.60%), geranyl acetate (1.68-3.44%), neryl acetate (0.67-3.03%), and thymol (0.19-4.76%) (Table 5). d-Limonene was the main component in all samples, with a composition ratio of 33.69%, 36.35%, and 45.42% for Sunkist, Jeramon, and Minimon, respectively. The d-limonene composition ratio of Minimon was 10% higher than that of the other two types of lemons. Limonene is the primary volatile compound found in lemon essential oil and accounts for its distinct citrus scent, which has a piney turpentine-like odor (Hong et al., 2017; Karabagias, 2017). Limonene is used in the food manufacturing industry as a flavoring agent to mask the bitter taste of alkaloids and as a fragrance material in perfumery and cosmetics (Del Nobile et al., 2008). The thymol ratios of Sunkist and Jeramon were low at 0.19% and 0.25%, respectively, but the thymol content of Minimon was quite high at 4.76%. Thymol has a rosemary- and thyme-like odor (Schreiner et al., 2020) and is used as a flavoring agent (Burdock, 2009; Suntres et al., 2015). Notably, thymol compounds have antimicrobial (Arfa, 2006; Del Nobile et al., 2008) and antioxidant activities (Horvathova et al., 2014) and are safe food preservatives (Beltrán Sanahuja et al., 2021). Other monoterpene hydrocarbons were also identified, including β-pinene, terpinolene, β-myrcene, and γ-terpinene. Such components have been previously studied in the volatile patterns of lemon essential oils (Lota et al., 2002).
The most abundant monoterpene alcohols of the three lemon varieties were linalool (2.02-2.48%), termine-4-ol (1.21-1.47%), and α-terpineol (3.23-3.63%). Linalool has diverse functionality, such as anticancer, and antioxidant properties, and several in vivo experiments have shown the effects of linalool on the central nervous system (Kamatou and Viljoen, 2008).
The main aldehydes detected in the lemon samples were neral (2.66-5.72%) and citral (3.75-7.64%), which showed the highest ratio in Jeramon. In addition, the esters identified in lemon samples were neryl isovalerate (2.19-6.33%), geranyl acetate (1.68-3.44%), neryl acetate (0.69-3.03%), and lavandulyl acetate (0.36–1.31%).
Our results showed that the volatile flavor components of Jeramon and Minimon had compositions similar to that of conventional lemons, with some components showing higher ratios. Minimon had a significantly higher limonene and d-thymol content than other varieties, suggesting that it might be valuable as a food additive, including flavoring.
4. Conclusions
The results of this study demonstrate that the new Korean lemon varieties, Jeramon and Minimon, are good sources of antioxidants and phytochemicals. Compared to Sunkist, the most consumed lemon variety in Korea, Minimon had more than twice as much β-cryptoxanthin, and the L-ascorbic acid content was more than 3–4-fold higher in both Minimon and Jeramon. Notably, Jeramon extract showed the highest antioxidant activity. Upon volatile analysis using the profiles of volatile components, high similarity among the three types of lemon samples was revealed, and terpenoids largely dominated the composition ratio. The ratio of d-limonene and thymol was much higher in Minimon than in the other varieties. More lemon varieties are expected to be grown in Korea, and this study on the physicochemical characteristics of Korean lemon varieties may be used as the basis for further breeding of domestic lemon varieties.