1. Introduction
Eagerness for good health; increase in single-person households, income per person, and the aged population; and changes in consumption trends favoring convenience have increased our interest in the use of fresh-cut fruits and vegetables as ready-to-eat foods, including salads, sprouted vegetables, and sliced fruit products. Changes in the demand for fresh-cut agricultural products have provided an opportunity for market expansion in Korea, including the development of new products in the agricultural industry (Choa, 2019; Kim et al., 2019). Relevant legislation by the National Agricultural Products Quality Management Service (NAQS) (Standards of Fresh-Cut Agricultural Products) in Korea defines fresh-cut agricultural products as “agricultural products, including vegetables, potatoes, and mushrooms, for cooking that are packaged and distributed after the process of washing, peeling, chopping, or slicing for convenient cooking” and states their quality standards (color, appearance, foreign substances, freshness, packaged state, foreign smell, etc.), packaging standards, and labeling (NAQS, 2022).
The market size for fresh-cut agricultural products is rapidly increasing in Korea owing to economic growth around the 1990s and an increase in individual income. In the early stage, there was an increase in the demand for fresh-cut agricultural products in the fields of food services and meal systems, and since 2000, the per individual consumption has also increased. The market size for fresh-cut fruits and vegetables in 2018 was estimated at approximately 808.9 billion KRW, with a steady increase in size (Kim et al., 2019; Park et al., 2020). Unlike general processed foods, fresh-cut agricultural products are produced, processed, and consumed without heat treatment for hygiene management and for preventing microbial contamination during production and distribution. These products undergo processing steps such as slicing and washing; as a result, changes in quality such as color change (browning), tenderizing (softening), and foreign smell rapidly occur (Lunadel et al., 2011; Park et al., 2001). Therefore, with the expanding market of fresh-cut agricultural products, processing and quality control techniques are warranted.
Fresh-cut fruits include sliced persimmon, mango, kiwi, banana, pear, apple, orange, papaya, and nuts as well as fruit cups mostly containing apple, kiwi, and melon (Kim, 2011). Apples are the most commonly cultivated fruits in Korea; most apples are consumed as fresh fruits (Jung and Kim, 2014). Statistical data from the Ministry of Agriculture, Food and Rural Affairs (MAFRA) suggest that the cultivation area and production of apples in Korea were 32,954 ha and 5.353 million tons, respectively, in 2019 (MAFRA, 2020). Fuji is the apple cultivar that is primarily produced and distributed in Korea; its production area has continuously increased. In the Cheongsong and Uiseong regions of Gyeongsangbuk-do, Korea, approximately 85% and 78% of farms, respectively, had cultivated apples in 2020 (Korea Agricultural Marketing Information Service, 2020). However, fresh-cut apples that undergo processing exhibit decreased storability and stability owing to browning caused by oxidation during peeling and slicing. In particular, the color of fresh-cut apples, a quality characteristic, is a critical quality indicator, warranting an investigation of the quality characteristics based on the usage of browning inhibitors (Hong et al., 2018). The use of browning inhibitors should facilitate the maintenance of freshness and induce no changes in flavor and color that are unique to each product. Browning of fresh-cut fruits and vegetables has been prevented by using reducing agents such as citric acid and ascorbic acid (AA) (Dong et al., 2000; Sapers and Miller, 1998). The extracts of licorice, green tea, dandelion, chrysanthemum, onion, apple, and citrus peel have been developed as browning inhibitors of natural substances in Korea (Jeong, 2012).
In the present study, we treated Fuji apples, which are commonly produced in Korea, with the browning inhibitors AA, calcium ascorbate (CA), and enriched citrus peel extract (Citrus unshiu Markovich, CuM) and elucidated the processing suitability and storability of fresh-cut apples during storage after processing by monitoring the changes in the physicochemical quality characteristics and antioxidant activities of fresh-cut apples.
2. Materials and methods
The apples used were the Fuji apples cultivated in Uiseong, Gyeongsangbuk-do, Korea, in 2022. Apples of even shapes were selected and used. The browning inhibitors CuM and AA were purchased from Serim Food (Bucheon-si, Korea) and CA was purchased from Sigma-Aldrich Co.. (St. Louis, MO, USA). Furthermore, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS), Folin-Ciocalteu’s phenol reagent, and all other reagents were purchased from Sigma-Aldrich Co..
Based on the Fresh-Cut Food Production Process, the selected apples were first washed with tap water to remove dust and foreign substances. Then, the apples were immersed in 100 ppm NaOCl for 1 min and subsequently in tap water for 30 s. Thereafter, the apples were sliced into eight pieces; each piece was immersed for 1 min in CuM, CA solution, and AA solution, each adjusted to 1%. This concentration was uniformly used based on the methods described by Kim et al. (2011) and Cho et al. (2012) for comparison. After natural drying for approximately 4 min, 120-150 g of apple samples were packaged in a common 500-mL polyethylene terephthalate container with a diameter of 98 mm and height of 130 mm; this container had a lid to allow airflow. The sample storage temperature was set at 4°C and 20°C. The storage period was 8 days for 4°C; quality evaluation was performed at 2-day intervals. On the other hand, the storage period was 4 days for 20°C; quality evaluation was performed every day.
The L value was measured using a colorimeter (CR-400; Konica Minolta, Tokyo, Japan). Weight loss rate was expressed as percentage (%), which was obtained by subtracting the weight at each storage date from the initial weight. Hardness was measured using a hardness analyzer (LF2050; Lloyd Instruments Ltd., Fareham, Hants, England). To measure soluble solid content, each apple sample treated with a browning inhibitor was ground using a homogenizer; fruit juice was used as the measurement sample. Measurements were performed using a digital Brix meter (HI96801; HANNA Instruments, Woonsocket, RI, USA). To measure pH, each apple sample treated with a browning inhibitor was ground using a homogenizer; fruit juice was used as the measurement sample. Measurements were performed using a pH meter (HM-30R; DKK-TOA Corporation, Tokyo, Japan). Total acidity was measured via malic acid conversion of the amount of 0.1 N NaOH consumed up to pH 8.3. Lastly, water content was measured using a water content analyzer (FD-720; Kett, Tokyo, Japan).
The DPPH radical scavenging activity of the samples was measured using the method described by Wahyono et al. (2020). Briefly, 50 μL of each sample treated with a browning inhibitor and 150 μL of 0.3 mM DPPH solution were mixed and allowed to react at 23°C for 30 min. Thereafter, the absorbance was measured at a wavelength of 517 nm. The absorbance of the sample was compared with that of the blank (control) to estimate the DPPH radical scavenging activity using the following equation:
A: Absorbance of the sample
B: Absorbance of the blank
The ABTS radical scavenging activity of the sample was measured using the method described by Wahyono et al. (2020). Briefly, 7.4 mM ABTS and 2.6 mM potassium persulfate were mixed and allowed to react for 24 h in a dark room. The ABTS solution was prepared by adjusting with PBS until the absorbance was 0.7±0.002 at 734 nm. To 20 μL of each sample treated with a browning inhibitor, 180 μL of the prepared ABTS solution was added. The mixture was allowed to react for 10 min in a dark room. The absorbance was measured at 732 nm. The radical scavenging activity was estimated using the following equation:
A: Absorbance of the sample
B: Absorbance of the blank
The FRAP assay was performed using the method described by Wahyono et al. (2020). The FRAP solution was prepared by mixing 0.3 mM sodium acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) dissolved in 40 mM HCl, and 20 mM ferric chloride at a ratio of 10:1:1 (v/v/v). Then, 50 μL of each sample treated with a browning inhibitor was mixed with 150 μL of the FRAP solution. The mixture was allowed to react in a dark room for 20 min. The absorbance was measured at 593 nm. For quantification, the standard curve was constructed using ferrous sulfate heptahydrate (FeSO4․7H2O).
TPC was measured using the method described by Stratil et al. (2006). Briefly, 50 μL of the sample and 50 μL of the Folin-Ciocalteu’s phenol reagent were allowed to react at room temperature for 3 min, followed by the addition of 150 μL of 2% sodium carbonate (Na2CO3). The mixture was allowed to react in a dark room for 2 h. The absorbance was measured at a wavelength of 760 nm. For quantification, the standard curve using gallic acid equivalent was constructed.
TFC was measured using the method described by Kim et al. (2021). To 20 μL of each sample treated with a browning inhibitor, 200 μL of diethylene glycol and 20 μL of 1 N NaOH were added. The mixture was allowed to react at 37°C for 1 h. The absorbance was measured at 420 nm. For quantification, the standard curve using naringin equivalent was constructed.
Each Fuji apple sample treated with a browning inhibitor was homogenized and placed in a 10-mL headspace vial. To prepare the internal reference material, 10 mL of 2-octanol was added to the sample. The mixture was incubated at 50°C for 30 min. The divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber (Supelco, St. Louis, MO, USA) containing 50 μm DVB layer and 30 μm CAR/PDMS layer was used to adsorb the VACs in the sample. The oven temperature was maintained at 40°C for 5 min and then raised to 105°C at 5°C/min and to 250°C at 20°C/min; finally, it was maintained at 250°C for 5 min. Quantification was performed via gas chromatography-mass spectrometric analysis using the Agilent 7010B QQQ GC–MS system (CA, USA). The column used was Agilent HP-INNOWAX (30 m×250 μm×0.25 μm). Analysis was performed in the selected ion monitoring mode.
All measurements were performed in triplicate and expressed as mean±SD. For statistical analysis, SPSS 20.0 (SPSS Inc., Chicago, IL, USA) was used to perform one-way analysis of variance with Tukey’s multiple comparison test as the post-hoc test to verify the significance of the experimental results. p<0.05 was regarded as statistically significant.
3. Results and discussion
Table 1 presents the changes in the physicochemical quality characteristics of Fuji apples based on the preprocessing steps. At each step, the L value of the processed fruits decreased compared with that of the original fruit. Furthermore, the soluble solid content was low at each step, except at the 30 min tap water immersion step, compared with the original fruit; however, the trends were not significant. Similarly, the total acidity and water content exhibited no consistent trend, despite statistical differences at each step. Moreover, hardness and pH exhibited no significant differences compared with the original fruit (p<0.05).
Data are expressed as mean±SD from three independent experiments (Tukey, p<0.05). Small letters indicate significant differences between stages. Raw means unprocessed fruit. Fruits were primarily washed under running tap water (Washing 1). Then, fruits were immersed in 100 ppm NaClO for 1 minute and then immersed in tap water again for 30 seconds (Washing 2). After that, fruits were cut into 8 pieces and soaked in 1% ascorbic acid solution for 1 minute. Finally, fruits were dried for about 5 minutes and then packaged.
Table 2 presents the changes in the physicochemical quality characteristics of Fuji apples after treatment with each browning inhibitor. Under 4°C storage conditions, the L value of all three groups (1% CuM, 1% CA, and 1% AA groups) tended to decrease based on the storage period; however, no significant differences were observed (p<0.05). Furthermore, no significant between-group difference was observed on the same storage date (p<0.05). Nevertheless, the weight loss rate significantly increased based on the storage period in all three groups (p<0.05); however, no significant between-group difference was observed on the same storage date (p<0.05). The soluble solid content significantly differed across the three groups, with a significant between-group difference on the same storage date (p<0.05); however, the trend was inconsistent. The pH increased from Day 2 of storage in the 1% CuM group and from Day 6 of storage in the 1% CA group; however, no pH changes were observed in the 1% AA group during the storage period. The between-group comparison suggested no significant trend. Hardness, total acidity, and water content exhibited no significant within-group or between-group differences after treatment with each browning inhibitor (p<0.05).
Under 20°C storage conditions, the L value of all three groups (1% CuM, 1% CA, and 1% AA) decreased based on the storage period. Although no significant difference was observed between the 1% CA and 1% AA groups (p<0.05), the L value of the 1% CuM group significantly decreased on Day 4 of storage compared with the other groups (p<0.05). On the other hand, the weight loss rate significantly increased in all three groups based on the storage period, although only the 1% CuM group exhibited significant differences on Day 4 of storage (p<0.05). Between-group comparison suggested that the weight loss rate of the 1% AA group was significantly low on Day 4 of storage (p<0.05). Moreover, hardness significantly decreased in the 1% CA and 1% AA groups on Day 4 of storage (p<0.05); however, no significant between-group differences were observed (p<0.05). The total acidity and water content exhibited no significant within-group or between-group differences, similar to the findings at 4°C storage (p<0.05).
Fig. 1 presents the changes in the antioxidant activity, TPC, and TFC of Fuji apples based on the preprocessing steps. Apples exhibit physiological properties such as antioxidant and antidiabetic effects owing to the presence of abundant levels of vitamin C, flavonoids, and polyphenols (Bang, 2015). In the present study, we elucidated the differences in the antioxidant activities of Fuji apples based on the preprocessing steps and browning inhibitor treatment.
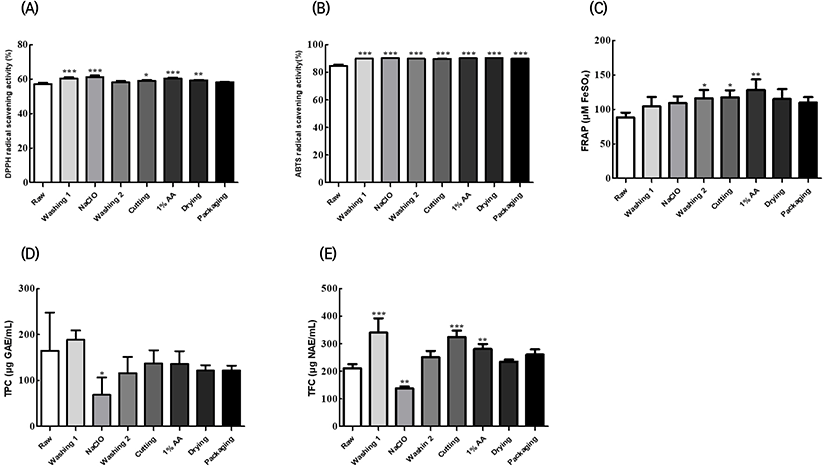
The DPPH radical scavenging activity is used to evaluate antioxidant activity based on the degree of discoloration of the free radical DPPH, which is purple in a stable state, when it reacts with an antioxidant material (Park et al., 2013). In this study, the DPPH radical scavenging activity was significantly higher in the apples treated with a browning inhibitor than in the original fruit at each step, except for the tap water immersion and packaging steps (p<0.05). On the other hand, the ABTS radical scavenging activity is used to evaluate antioxidant activity based on the discoloration of the ABTS cations, which are produced from the reaction between ABTS and potassium persulfate, when they react with an antioxidant material (Part et al., 2013). The ABTS radical scavenging activity was significantly higher in the treated fruits than in the original fruit at each step (p<0.05). The FRAP assay is a method to determine the antioxidant activity based on the decrease in the ferric tripyridyltriazine (Fe3+–TPTZ) complex into ferrous tripyridyltriazine (Fe2+–TPTZ) when it reacts with an antioxidant material (Park et al., 2013). The FRAP of the processed fruits was significantly higher than that of the original fruit at the tap water immersion, slicing, and 1% AA treatment steps; however, no significant differences were observed after the drying step (p<0.05). Polyphenols exhibit various types of bioactivities, including reactive oxygen species removal; furthermore, the bioactivities of flavonoids, secondary metabolites with a polyphenol structure, include anticancer and anti-inflammatory effects (Kim and Kim, 2021). At the 100 ppm NaClO treatment step, the TPC and TFC of Fuji apples were distinctly lower than those of the original fruit.
Fig. 2 demonstrates the changes in the antioxidant activities, TPC, and TFC of Fuji apples after treatment with each browning inhibitor.
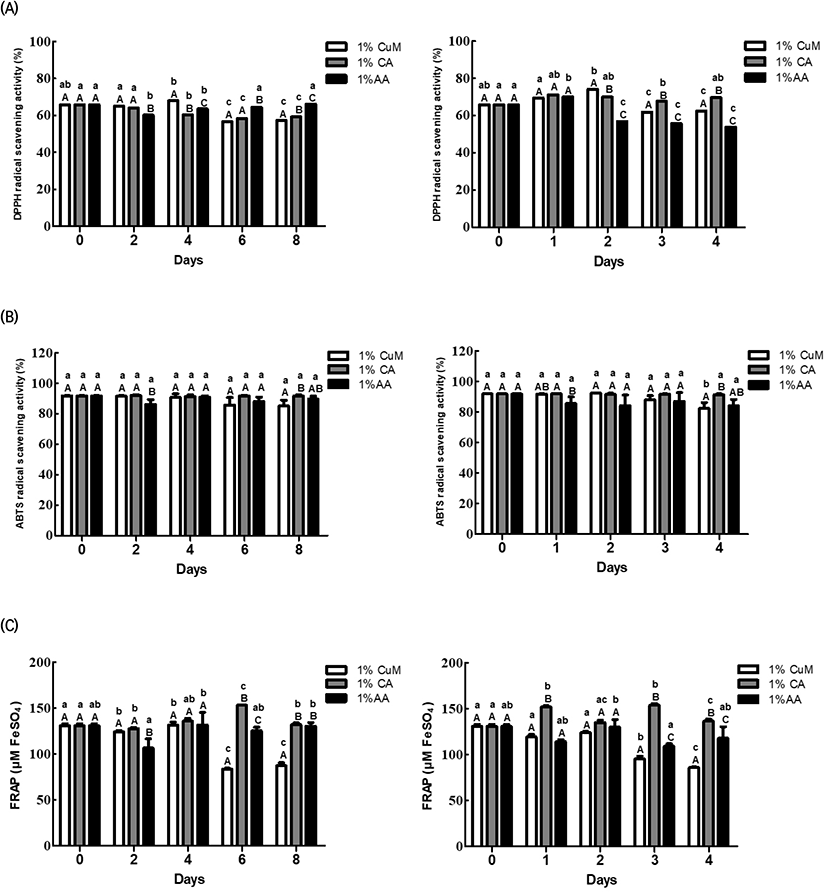
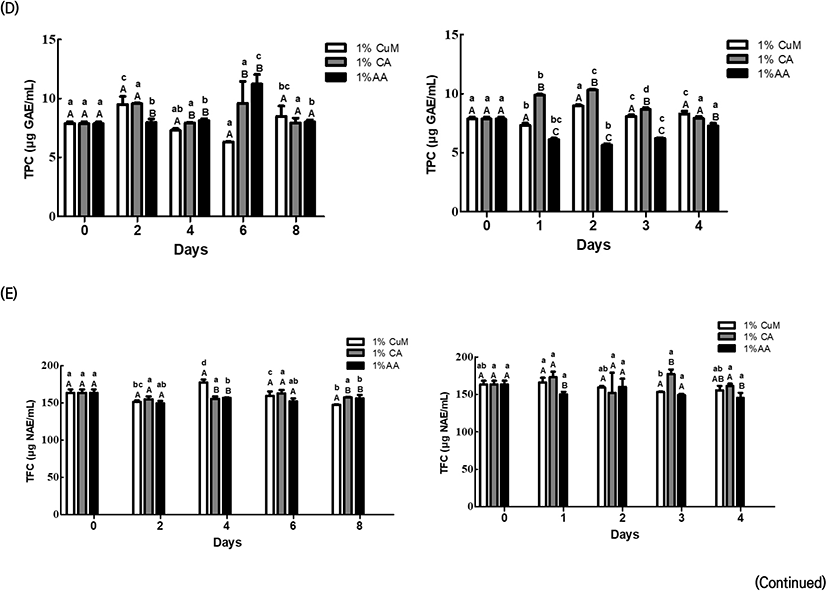
On Day 2 of storage at 4°C, the browning inhibitor treatment groups exhibited a significant between-group difference based on storage date for the 1% CuM group (p<0.05); however, the trend was inconsistent. On the other hand, no significant within-group difference was observed based on the storage period for each browning inhibitor treatment group (p<0.05). Between-group comparisons suggested significantly lower values in the 1% AA group than in the other two groups on Day 2 of storage at 4°C and on Day 1 of storage at 20°C, although the differences subsequently disappeared (p<0.05).
From Day 6 of storage 4°C, the 1% CuM group exhibited distinctly lower values compared with the other groups. Furthermore, FRAP considerably decreased in the 1% CuM and 1% AA groups on Day 1 and after Day 3 of storage at 20°C. On the other hand, the 1% CA group maintained relatively steady values at both temperature conditions. TPC significantly differed among the three browning inhibitor treatment groups at each storage date for storage at 4°C (p<0.05), although the trend was inconsistent. However, TPC markedly decreased in the 1% AA group from Day 1 of storage at 20°C. TFC was significantly lower in the 1% CuM group than in the other groups on Day 8 of storage at 4°C and in the 1% AA group from Day 3 of storage at 20°C (p<0.05).
AA, also called vitamin C, is a widely known antioxidant agent (Arrigoni et al., 2002) and is used as a material to prevent browning (Nam et al., 2021). AA treatment in the preprocessing step was anticipated to inhibit apple browning and improve its antioxidant activity; however, the L value and antioxidant activities measured in the present study did not significantly differ before and after AA treatment.
Park et al. (2013) have reported that the fruits of the Citrus genus contain abundant metabolites, including naringin and neohesperidin; these metabolites exhibit antioxidant effects. Furthermore, AA and CA are well-known browning inhibitors for apples and pears, with reliable effects (Allegra et al., 2022). In the present study, we treated apple samples with CuM, CA, and AA to assess processing suitability by evaluating physicochemical quality characteristics and antioxidant activities. At 4°C storage, the L value did not significantly differ among the three browning inhibitor treatment groups. In contrast, at 20°C storage, the L value of the CuM group significantly decreased compared with that of the other two groups. This finding, in agreement with that of Park et al. (2013), may be owing to the discoloration caused by the color of the diluted solution of the CuM extract. However, further investigations are warranted because a previous study has reported that polyphenol oxidase activity can be inhibited by treating with a citrus peel extract in a concentration-dependent manner (Chang et al., 2011).
High concentrations of ethyl acetate after storage are strongly related to off-flavor, but at low concentrations, it composes the apple aroma (Schmidt et al., 2019). Additionally, the accumulation of hexanal and ethyl acetate was identified as potential volatile biomarkers related to damaged apples (Lin et al., 2021). Table 3 presents the volatile compounds in apple juices of Fujiapples treated with anti-browning agents during the storage period. At storage 4°C, the group treated with 1% CuM exhibited the highest levels of ethyl acetate and hexanal.
Data are expressed as mean±SD from duplicate experiments.
Hexanol is associated with odor of freshly cut, green grass (Aaby et al., 2002). However, contrary to the previous information, it was observed that hexanol was also detected at the highest level in the group treated with 1% CuM. Additional experiments are required to obtain more accurate results.
4. Conclusions
In the present study, the processing suitability and storability of fresh-cut Fuji apples were evaluated based on their physicochemical quality characteristics and antioxidant activities during the preprocessing steps and storage after the treatment with the browning inhibitors 1% CuM, 1% CA, and 1% AA. We observed that in the production of fresh-cut Fuji apples, the DPPH and ABTS radical scavenging activities and FRAP did not significantly differ across the preprocessing steps; on the other hand, TPC and TFC markedly decreased in the NaClO treatment step. The storage of fresh-cut Fuji apples with browning inhibitor treatment at 4°C led to a decrease in L value and hardness and an increase in weight loss rate based on the storage period in all three treatment groups. Furthermore, pH increased on Day 2 of storage in the 1% CuM group and on Day 6 of storage in the 1% CA group; however, the pH of the 1% AA group did not change during the storage period. The DPPH radical scavenging activity was mostly high in the 1% AA group at storage at 4°C and in the 1% CA group at storage 20°C. Furthermore, FRAP was maintained at a relatively steady level in the 1% CA group. Taken together, the results of the quality characteristics and antioxidant activities of fresh-cut fruits at each processing step and during storage can be used as basic data for industries. These data may increase the reliability of quality enhancement by improving the production and distribution conditions of fresh-cut agricultural products and contribute to expanding farm income and improving competitive power via the stabilized consumption of fresh-cut agricultural products.
