1. Introduction
Kiwis (Actinidia deliciosa) belongs to the family Actinidiaceae, and genus Actinidia, and contain vitamins, dietary fibers, minerals, and phytochemicals (such as flavonoids, carotenoids, polyphenol, and lutein) (Satpal et al., 2021). Most kiwis cultivated in Korea are Hayward cultivars, which are preferred for wine production because of their large size. Persimmon fruit (Diospyros kaki) also contains glucose, fructose, minerals, vitamins C, A, and B, epicatechins, and large amounts of tannins (Joo et al., 2011; Lee et al., 2012). The fruit contains a number of aromatic hexnalas and organic acids, such as malic acid, dietary fibers, vitamins C and E, minerals, carotenoids, polyphenols, and flavonoids (Cho et al., 2004).
Wine is the product of a complex process involving yeasts and grape must. The sales rate of wine in Korea has increased significantly over the past 20 years, in line with economic improvements and the changes in the drinking culture of Korea. However, the Korean domestic wine industry is relatively new, and most wines consumed in Korea are imported from other countries (Hong and Park, 2013). To mitigate this demand, several strategies, such as using other than current yeast strains, fruits other than grapes, and mixing different fruits, are needed.
Aroma is one of the most important quality factors in wine and is one a the key determinants of consumer acceptance (Sanchez-Palomo et al., 2010). More than 1,300 volatile compounds including alcohol, esters, acids, aldehydes, isoprenoids, lactones and ketones provides the complex sensory characteristics of wine (Lin et al., 2019; Yan and Dong, 2019). In the past, strains of S. cerevisiae were chosen for their enological properties and have been used for fermentation strains for carrying out alcoholic fermentation in wine production (Suzzi et al., 2012). The composition and the sensory qualities of the resulting wines are attributed to the diversity of S. cerevisiae strains and to their widespread use in fermentation (Torrens et al., 2008). S. cerevisiae strains hydrolyze sugar and convert in alcohols, acids, and CO2 during alcohol fermentation. The produced alcohol and organic acids stress the yeast (Caridi et al., 1999). Yeast fermentability is of yeast was affected by various environmental stresses such as high concentrations of sugars, alcohols, and acids (Aguilera et al., 2006). Thus, various stress factors, depending on the fermentation conditions, affect the fermentation strains. Therefore, optimal strains must be selected through appropriate experiments and used them for fermentation purposes (Kim et al., 2013; Zuzuarregui and Del Olmo, 2004).
In this study, we evaluated the tolerance of yeasts isolated from various fruits against sucrose, alcohol, pH, and potassium metabisulfite was evaluated. Among the isolated strains, kiwi-persimmon mixed wine (KPMW) was fermented by five yeast strains with excellent tolerance. The physicochemical properties; levels of sugars, organic acids, and volatile flavor compounds; sensory properties; browning degree; and antioxidant properties of the KPMWs made with these strains were analyzed. Based on the obtained results, we tried to select yeasts suitable for the production of high-quality wines were selected.
2. Materials and methods
Fruits and fermented vinegar from kiwi, persimmon, apple, pear, watermelon, and grape were purchased from a domestic supermarket (Jinju, Korea), together with materials for producing kiwi-persimmon mixed wine were also purchased from the same place. The commercial S. cerevisiae KCCM 12615 yeast strain was obtained from the Korean Culture Center of Microorganisms (Seoul, South Korea). Other reagents and YPD yeast culture media (5.0 g of yeast extract, 5.0 g; peptone, 5.0 g; and dextrose, 20 g) used in this study were of analytical grade. 2,2’-azino-bis-(3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ), Ferric-Chloride’s, folin-ciocalteu phenol reagent, glacial acetic acid, potassium persulfate, and sodium acetate were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). HPLC-grade water, methanol, and acetonitrile were purchased from Fisher Scientific (Seoul, South Korea). All the other reagents were of analytical grade.
Each fruit was pulverized and placed in 1.0 L glass bottles for two weeks for alcohol fermentation. Fermented liquid samples were then subjected to 10-fold dilutions. Next, 100 μL volume of each diluted (103 to 106) sample was spread on a chloramphenicol -containing PDA plate and incubated at 30°C for 48 h. Several different forms of yeast colonies were observed in on the plates, of which a 100 colonies were isolated and cultured in a YPD liquid medium. The cultured yeast strains were then separated and selected based on their resistance to sucrose, acid, alcohol, and potassium metabisulfite resistances. Highly resistant yeast strains were used for kiwi and persimmon fermentations. To identify the highly resistant yeast strains, all strains were cultured in 100 mL flasks for 30±6 h at 30°C. The cells were collected after centrifugation at 13,000 ×g for 5 min. Genomic DNA extraction, 26S rRNA gene amplification, and gene cloning and sequencing were performed as previously described by Haque et al. (2015). Nucleotide sequences were deposited in the GenBank database under the accession numbers KU499842-KU499846.
The sucrose tolerance of the selected yeast strains was tested against various levels of sucrose. The yeast strains (5% v/v) were inoculated with 0%, 20%, 30%, and 40% sucrose in YPD broth media and cultured at 30°C for 48 h, respectively. To determine the alcohol tolerance, the yeast strains were similarly cultured in YPD broth media with at 0, 5, 10, and 15% alcohol. To determine the acid tolerance, the yeast strains were grown in YPD broth media at pH 6.5, 5, 4, and 3. Finally, to determine the sulfur (K2S2O5) tolerance, the yeast strains were grown in YPD broth media containing 0, 100, 200, and 400 mg/mL sulfur. The strain’s growth was confirmed by calculating the optical density of the cultures at 600 nm.
Kiwi and persimmon fruits were washed three times with running tap water and dried to remove surface water. Next, they were then ground in a blender (38BL54, WARING Co., USA) to extract the fruit juices. Juice solutions were prepared with rapidase (0.2%) and plum (Japanese Aprico; 5%), and heated at 45°C for 2 h. Subsequently sucrose was added to make 24 °Brix solutions. These sweetened kiwi and persimmon solutions were mixed at a 7:3 ratios and sterilized at 65°C for 30 min. The cooled sterilized juices were inoculated with the selected yeast strains at 2.5% and supplemented with ammonium sulfate (0.5 g/L). The fermentation was allowed at 15°C for 0-14 days, then at 20°C for 14-35 days. Samples were collected after 0, 14, and 35 days of fermentation.
The pH of the supernatants was measured using a pH meter (model 3510; Jenway, UK). Solution acidity was measured by titration with a 0.01 N NaOH solution and displayed as lactic acid (%) according to methods previously described by Joo et al. (2011) and Hwang et al. (2018).
The sugar contents of the collected samples was measured using a refractometer (Sccharometer, WSRO-90, Atago Co., Tokyo, Japan). The glucose concentrations in the centrifuged wine samples were measured using the DNS method (Miller, 1959).
The collected samples were serially diluted in sterile distilled water, plated on YPD agar plates containing 15 mg/L chloramphenicol and incubated at 30°C for 48 h. The number of formed yeast colonies formed is expressed as of log CFU/mL. To determine the alcohol content, a 50 mL sample was aliquoted into a 500 mL Erlenmeyer flask and diluted with 100 mL of distilled water. The mixture was boiled and reduced to 25 mL and diluted 1:1 with adding an additional 25 mL of distilled water. The alcohol content was measured using an alcohol hydrometer (MT-830; Atago Co., Tokyo, Japan) (Cho and Joo, 2014).
The degree of browing and soluble phenolic contents of the wine samples were measured using a spectrophotometer (Spectronic 2D, Thermo Co., Petaluma, CL, USA) at 420 nm and 750 nm, respectively, and expressed in absorbance units according to the methods previously described by Joo et al. (2011).
The organic acid and free sugar contents of the wines were determined according to methods previously described by Cho and Joo (2014).
Volatile compounds in the wine samples were extracted and analyzed using solid phase-micro extraction (SPME) and a headspace (Autosampler, HS-7697A, Agilent Technologies, Santa Clara, CA, USA) according to methods previously described by Cho et al. (2017) and Song et al. (2019). The samples (5 mL) samples were sealed in a 20 mL glass vial with an aluminum cap; subsequently, 100 μm polydimethylsiloxane (PDMS) 100 rpm in fiber was added and the samples were adsorbed at 100°C for 8 min.
The antioxidant activities as well as ABTS (2,2’-azino-bis-(3-ethyl-benzothiazoline-6-sulfonic acid) diammonium salt) and DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activities of the samples were measured, and an antioxidant power assay was performed by following the methods described by Joo et al. (2011) and Hwang et al. (2018).
All analyses were expressed as the mean±standard deviation (SD) with tree replicates measurements. Significant differences among samples in the tolerance tests were confirmed by Tukey’s multiple test (p<0.05) using the Statistical Analysis System (SAS) software (ver. 9.4; SAS institute, Cary, NC, USA).
3. Results and discussion
One hundred yeast colonies were isolated from the culture plates and all strains were tested for their resistance to pH, potassium metabisulfite, and high concentration of sucrose and alcohol to select the yeast strain appropriate for wine fermentation. Five yeast strains Y4, Y10, Y28, Y78, and Y81, exhibited higher tolerance compared with the S. cerevisiae KCCM 12615 (Fig. 1).
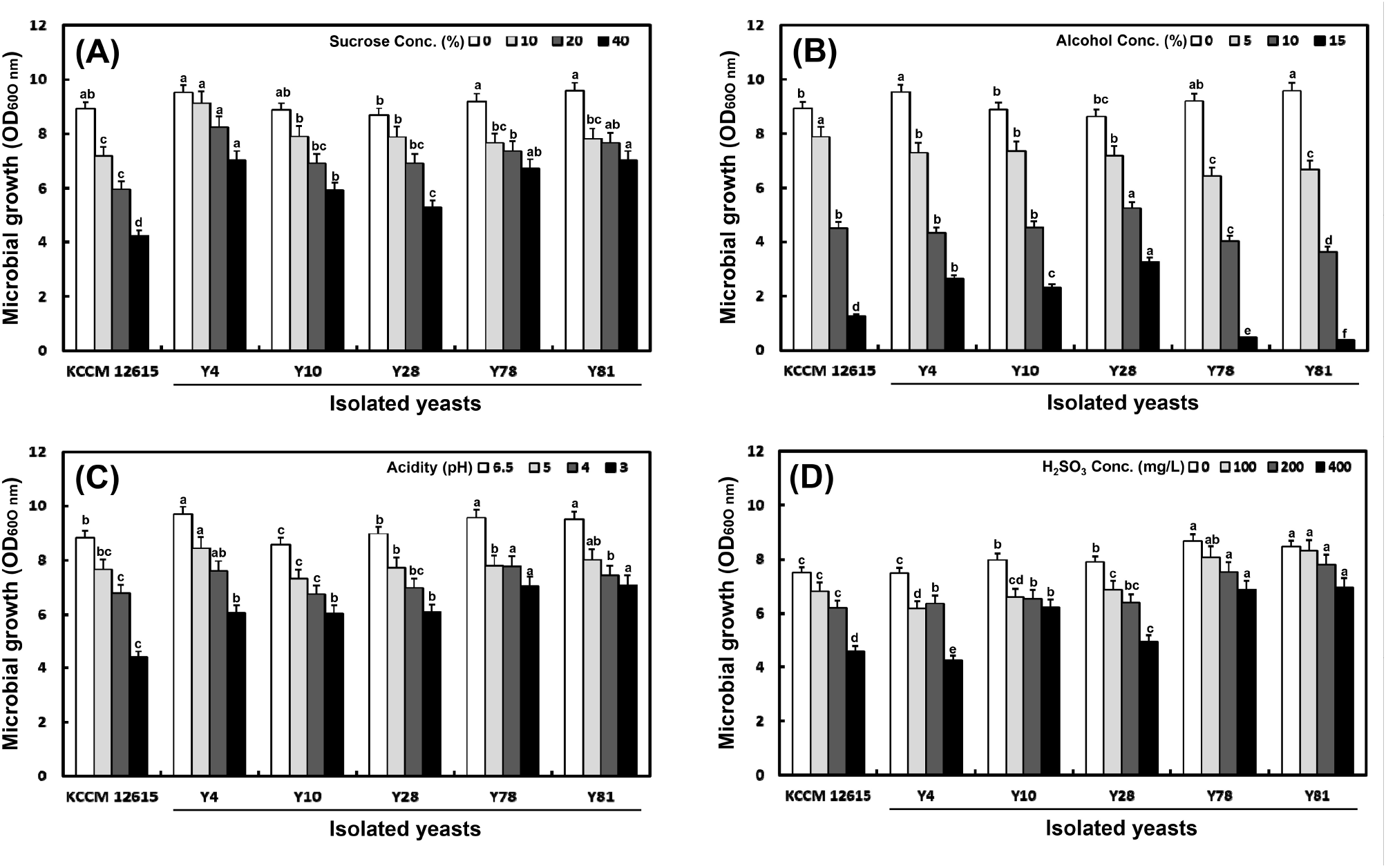
As shown in Fig. 1(A), the yeasts density (OD600 nm) generally decreased with increasing concentrations of sucrose. At a sucrose concentration of 40%, the yeast densities were 1.66-, 1.34-, 1.25-, 1.59-, and 1.66-folds higher than those of S. cerevisiae KCCM 12615. Similarly, yeast density decreased with increasing alcohol concentrations (0-15%) (Fig. 1(B)). At an alcohol concentration of 5%, the selected yeast strains exhibited slightly lower densities than S. cerevisiae KCCM12615. At the same alcohol concentration, the yeast strains Y28, Y4, and Y10 exhibited markedly higher growth rates than S. cerevisiae KCCM 12615, Y78, and Y81. As shown in Fig. 1(C), the yeast density decreased with lower acidity (i.e., lower pH). Importantly, the selected yeast strains exhibited higher growth rates than S. cerevisiae KCCM 12615 under different pH conditions. At pH 3, the Y78 and Y81 strains exhibited 1.59- and 1.61-folds higher growth rates, respectively, than the control S. cerevisiae KCCM 12615. Potassium meta-bisulfite preservatives are typically added during wine fermentation to prevent the growth of harmful pathogenic bacteria. However, they may interfere with yeast growth at high concentrations. Therefore, 0-400 mg/mL potassium meta-bisulfite was added to the YPD broth cultures to evaluate its effect on yeast growth (Fig. 1(D)). Y4 growth was lower than that of the S. cerevisiae KCCM12615 at 100 to 400 mg/mL potassium meta-bisulfite. However, the growth rates of other four isolates were moderately higher than those of S. cerevisiae KCCM12615 and Y4. In particular, the growth of the Y78 and Y81 strains was 1.5- and 1.52-folds higher than that of S. cerevisiae KCCM 12615 in the presence of 400 mg/mL potassium meta-bisulfite. Five S. cerevisiae yeasts, Y4, Y10, Y28, Y78, and Y81, exhibited higher tolerance to sucrose, alcohol, pH and potassium metabisulfite during kiwi-persimmon mixed wine (KPMW) production compared with the S. cerevisiae KCCM 12615 yeast strain.
As presented in Table 1, strains Y4, Y10, and Y28 were isolated from golden kiwis, loquats, and wild berries, respectively. The Y78 and Y81 strains were isolated from grapes. Molecular biology techniques provide a simple and rapid method for differentiating and identifying yeasts based on their genetic backgrounds (Haque et al., 2015). Interestingly, the 26S rRNA gene sequences of these yeast strains exhibited 99% similarity to S. cerevisiae D3C. Based on this molecular characterization, these five isolates were identified as S. cerevisiae species. The use of yeasts has progressively evolved from naturally occurring yeast to starter cultures of selected strains (most of which belong to S. cerevisiae species) (Mostert and Divol, 2014). Therefore, the selected yeasts were further tested in micro-fermentation assays to evaluate the strain-specific impact on the physicochemical properties, free sugar and organic acid contents, volatile flavor compounds, sensory properties and antioxidant activities.
After fermentation, the physicochemical properties of wines made with S. cerevisiae KCCM 12615 were analyzed and compared with those of wines made with the selected strains (Table 2). The assays revealed that the pH values of the KPMWs produced by the isolates were slightly higher (range 3.71-3.78) compared with those of the wines prepared using S. cerevisiae KCCM 12615. However, the acidity was slightly lower at pH 1.89 and 1.87 for the wines produced by the Y10 and Y28 strains. In fact, the lowest sugar content in the wines prepared by S. cerevisiae KCCM12615 was 9.0 °Brix, which was similar (9.2-10.0 °Brix) to those of the wines produced by the Y10, Y28, and Y4 strains. However, the sugar content was 1.57- and 1.73-folds higher in the wines prepared with the yeast strains Y81 and Y78, respectively, compared with that in the wine from S. cerevisiae KCCM 12615. In the KPMWs, the reducing sugar content was the lowest (5.79 °Brix) in the wine produced by S. cerevisiae 12615 strain, which was similar (6.27 °Brix) to that produced by Y28, and markedly lower (7.02 and 13.54 °Brix) than those produced by the Y10 and Y4 strains, respectively. Furthermore, alcohol production (10.0-10.2%) was similar in the wines produced by the S. cerevisiae KCCM 12615, Y4, Y10, and Y28 strains, whereas the Y78 and Y81 strains exhibited lower alcohol contents (6.0-7.0%). Additionally, the viable cell numbers (log CFU/mL) were 8.39, 7.96, 7.54, 7.94, 8.04, and 8.11 in the wines produced by the by S. cerevisiae KCCM 12615, Y4, Y10, Y28, Y78 and Y81 strains, respectively. Thus, that no significant differences were observed between the S. cerevisiae KCCM12615 and Y28 strains in terms of pH, acidity, brix, reducing sugar, alcohol content, and viable cell numbers. The alcohol levels were relatively lower in the wines produced by the Y78 and Y81 strains, and the brix and reducing sugar contents were remarkably higher that those of commercial wines.
In a related study, cherry wine made by S. cerevisiae D254 and EC1118 exhibited alcohol concentrations of 10.24 and 10.30 and, pH levels of 4.08 and 4.11, respectively (Sun et al., 2015). In another study, grape wine made from S. cerevisiae W-3 exhibited pH 3.42, °Brix 7.0, and an alcohol level of 11.7 (Hong and Park, 2013).
As presented in Table 3, all fermentations, except for those of the Y4, Y10, and Y28 strains, resulted in a residual sugar content of less than 3.0 g/L. In fact, the fermentation by the Y78 and Y81 strains resulted in higher concentrations of free glucose and fructose. This indicates that the Y4, Y10, and Y28 strains consumed most of the free sugars during wine fermentation, thereby resulting in lower concentrations of free sugars in the KPMWs. When grape skins are macerated into grape juice, saccharides are produced from polyphenols during long aging processes (Siren et al., 2015). Additionally, the fermentation of hemicellulose releases saccharides (Wirth et al., 2012). In this study, KPMWs made from Y78 and Y81 exhibited high amounts of fructose (70.13 and 77.64 g/L, respectively). Because of the high free sugar content in the Y78 and Y81 generated KPMWs, these KPMWs were considered the sweetest of the studied wines.
Acids impart stability to wines and are important for preserving their organoleptic qualities and colors. Their importance in preventing undesirable byproduct formation during fermentation is well-known (Cho and Joo, 2014). In this study, all KPMWs exhibited low levels of fumaric, succinic, and glutamic acids and high levels of citric, tartaric, and malic acids. In particular, the total organic acid contents were estimated as 10.85, 11.52, 11.83, 11.48, 15.73, and 14.49 g/L in the KPMWs made from the S. cerevisiae KCCM12615, Y4, Y10, Y28, Y78, and Y81 strains, respectively. This indicates that KPMWs made from Y78 and Y81 were more acidic compared with other KPMWs. The sweetness and acidity of wine are based on the sugar and organic acid concentrations, respectively (Siren et al., 2015). KPMWs made from Y78 and Y81 contained higher concentrations of free sugars and organic acids. Tartaric and malic acids typically account for the overwhelming majority of fixed acids in wines, whereas pyruvate and other nonvolatile acids, such as succinic, oxalic, citric, and lactic acids, may be present in minor concentrations (Cho and Joo, 2014), which was also observed in the current study.
As presented in Table 4, 27 volatile compounds were detected, among which, esters were the most prevalent compounds. High levels of ethyl acetate were found in KPMWs made from the Y10 and Y28 strains. Isoamyl acetate, which is known to contribute to aromaticity, was present in moderate amounts in wines made from all the tested yeast strains. In additionally, lower quantities of isobutyl formate were detected. However, KPMW prepared from S. cerevisiae KCCM 12615 contained higher levels of isoamyl formate, ethyl butanoate, isoamyl acetate, ethyl hexanoate, and ethyl hexanoate esters. Ethyl isovalerate was the only ester found in KPMW made from Y4. Ethyl octanoate was detected at higher concentrations than the other esters in KPMW made from S. cerevisiae KCCM 12615. Low concentrations of ethyl the other hexanoate were detected in KPMWs prepared from S. cerevisiae KCCM12615, Y4, Y10, and Y28.
Ethyl esters, isoamyl acetate, ethyl octanoate and ethyl hexanoate contribute fruity and floral flavors to the sensory characteristics of wines (Caliari et al., 2015). Among the five alcohols detected, isoamyl alcohol was the most abundant in KPMWs made from the Y10 and Y28 strains. Interestingly, the studied KPMWs prepared from all yeast strains except Y81 exhibited lower concentrations of phenylethyl alcohol compared with other alcohols. However, 1-pentanol (which contributes to sweet and vanilla flavors) was found at higher concentrations only in KPMWs made from Y78 and Y81. Isoamyl alcohol may also contribute to aromaticity. However, the positive or negative influence of higher alcohols is dependent on the type of wine and its aroma (Ugliano and Henschke, 2010). 2-Phenyl-ethanol is a yeast metabolite that has a floral aroma, with rose notes (Sanchez-Palomo et al., 2010).
Furfural was the only aldehyde found in KPMWs made from S. cerevisiae KCCM12615. 4-Hydroxy-2-butanone was detected at 6.3-fold higher concentrations in KPMWs prepared from Y4 compared with in KPMWs prepared from S. cerevisiae KCCM12615. Additionally, 3-methyl-tetrahydro-furan was detected in negligible amounts in KPMW made from S. cerevisiae KCCM12615. Acetaldehyde is the most abundant aldehyde found in wine. However, acetaldehyde was not detected in the produced KPMWs; these results agreed with those of Pereira et al. (2014). Furfural is produced from the thermal degradation of hexose sugars. However, none of these aldehydes or ketones were detected in KPMWs prepared from the Y10 and Y28 strains. Two volatile cyclic compounds, cis-1,2-dimethyl cyclopentane and 2-formyl-1-methylpyrrole, were detected in negligible amounts in KPMW made from S. cerevisiae KCCM12615. Similarly, 1-trans-2-dimethylcyclopentane was detected in negligible amounts in KPMW made from Y28. Two sulfur compounds, propene sulfide and 2,4-dimethyltetrahydro-2H-thiopyran 1,1-dioxide, were detected in KPMW prepare from S. cerevisiae KCCM12615. These results suggest that strains Y4, Y10, Y28, Y78, and Y81 did not produce any sulfur compounds during wine fermentation. Volatile sulfur compounds in wines are typically responsible for unpleasant odors, even when present in trace amounts. Their presence in wines is typically associated with enzymatic or non-enzymatic degradation of sulfur containing compounds, particularly amino acids (Mestres et al., 2000). Furthermore, all wines had ethyl butanoate esters, while the KPMW made from Y10 and Y28 exhibited higher concentrations of ethyl acetate esters. Ethyl esters contribute to the general quality of wines positively and are responsible for their fruity and floral sensory properties (Perestrelo et al., 2006). The KPMWs made from S. cerevisiae KCCM 12615, Y4, Y78, and Y81 showed lower acceptability owing to the higher concentrations of 4-hydroxy-2-butanone ketones. Duarte et al. (Duarte et al., 2010) reported that 3-hydroxy-2-butanone might influence wine aroma, thus contributing to lower acceptability.
The degrees of browning in the tested wines were similar. The TPCs were estimated at 1.1 g/L for KPMWs made from the S. cerevisiae KCCM12615, Y4, Y10, and Y28 strains, whereas they were the highest (1.57 and 1.42 g/L) for the wines made from the Y78 and Y81 strains, respectively. The DPPH and ABTS radical scavenging activities and the FRAP assay values were 77.94%, 75.73%, and 0.97 for the wines produced by Y78, and 79.31%, 78.37%, and 0.97 for those produced by Y81, respectively. The lowest DPPH and, ABTS radical scavenging activities and FRAP assay values were detected in the wine produced by the Y10 strain. Higher values were detected in wine produced using the Y4 strain than in wine made from S. cerevisiae KCCM12615. These antioxidant activities were lower in KPMW produce by the Y28 strain (Table 5). The inhibition percentages were 84.01% for Kalecik Karasi, 81.34% for Cabernet Sauvignon and 19.16% for Narince. Katalinic et al. (2004) reported that the inhibition percentage of wines diluted with water is 54.6-82.2% for red wines and 10.7-16.2% for white wines.
4. Conclusion
KPMWs made from Y4, Y10, Y28, Y78, and Y81 exhibited significant differences in tolerance, physicochemical properties, free sugar, and organic acid contents, volatile compounds produced, sensory analyses, and antioxidant analyses. The Y28 strain showed high tolerance to sucrose, alcohol, pH, and potassium metabisulfite compared with the control strain (S. cerevisiae KCCM12615). However, in addition to the volatile flavor compound profiles, no significant differences were not observed compared with the KPMWs made from S. cerevisiae KCCM 12615. Importantly, the absence of volatile sulfur compounds in KPMW made from the Y28 strain contributed to its acceptability. Therefore, Y28 is a promising yeast strain for producing high-quality wines.
