1. Introduction
King oyster mushrooms [Pleurotus eryngii (De Canolle ex Fries) Quel.] phylogenetically belong to the phylum Basidiomycota, the order Agaricomycetes, the family Pleurotaceae, and the genus Pleurotus. Their primary natural habitats are the subtropical regions or dry grasslands in southern parts of Europe and northern parts of Central Asia and Africa (Ahn et al., 2006). In South Korea, a cultivation technique was developed by the National Institute of Agricultural Sciences (NAS) of the Rural Development Administration in collaboration with the Gyeongsangnamdo Agricultural Research and Extension Services (GNARES) for distribution across farms in May 1997. The consumption of king oyster mushrooms has steadily increased owing to their unique flavor and delicate texture (Ryu et al., 2006; Ryu et al., 2007). As a result, the cultivation area and number of farms for king oyster mushrooms have significantly increased. Moreover, since 2007, the respective production has been the highest among mushrooms cultivated in South Korea (Ha et al., 2014).
Overseas exportation of king oyster mushrooms increases each year and accounted for USD 25.867 million (53%) of the total mushroom exportation at USD 48.985 million in 2020, leading the edible mushroom export in South Korea [Korea Agricultural Trade Information (KATI), 2022; National Institute of Horticultural and Herbal Science (NIHHS), 2022]. Currently, the exportation of king oyster mushrooms mostly involves marine transportation, requiring 21 day to Australia, and 28 day to the U.S. and Europe (Choi et al., 2012; NIHHS, 2021). However, these mushrooms lack an outer protective layer, unlike other horticultural crops. Moreover, the high water content and active respiratory activity cause rapid quality reduction due to digestive enzymes and microbial growth after harvest (Guo et al., 2023). Thus, a post-harvest treatment technique that can maintain quality during marine transportation is necessary to ensure the commercial values of king oyster mushrooms.
The post-harvest treatment of mushrooms has applied chemical methods, such as antimicrobial agents, anti-browning agents, edible coating agents, ozone treatment, and electrolyzed water treatment, and physical methods, such as drying and non-thermal treatment with ultrahigh pressure, plasma, pulse electric field, and food irradiation. The key mechanism of these techniques is known to enhance storability by inhibiting microbial growth and enzymatic activity (Castellanos-Reyes et al., 2021). Food irradiation has high penetrability and can be applied to packaged products as an effective non-thermal treatment to prevent secondary contamination (Kim et al., 2013). The domestically available irradiation sources for food are gamma rays (1.17 and 1.33 MeV) from radioactive isotopes (60Co) and X-rays (≤5 MeV or ≤7.5 MeV) and electron beams (≤10 MeV) generated by electron accelerators. Among the irradiation sources, gamma rays are suitable for the treatment of large volumes of final products due to their high penetration and uniformity. Thus, gamma irradiation accounts for the largest proportion of the global radiation sterilization market (Ashraf et al., 2019; Indiarto et al., 2020). Food irradiation is toxicologically, nutritionally, and microbiologically safe up to 10 kGy, and recently, 52 countries have permitted food irradiation for more than 250 products (Roberts, 2014). For mushrooms, in particular, a maximum irradiation dose of 1-3 kGy is permitted in overseas countries (Argentina, Hungary, Israel, Mexico, Poland, and China) for improved storability and sanitization (Akram and Kwon, 2010) but in South Korea, maximum irradiation dose of 1 kGy is permitted [Ministry of Food and Drug Safety (MFDS), 2023]. Although many studies have investigated button mushrooms (Agaricus bisporus) (Beaulieu et al., 1999; Dong et al., 2022; Duan et al., 2010; Koorapati et al., 2004; Mami et al., 2014; Wani et al., 2009), king oyster mushrooms have not been sufficiently investigated yet. Thus, this study aimed, first, to improve the storability of king oyster mushrooms through gamma irradiation as a post-harvest treatment, and second, to determine the optimal irradiation dose to maintain the commercial quality of king oyster mushrooms during storage period of 28 days.
2. Materials and methods
King oyster mushroom variety No. 2 was purchased from Korea Green Mushroom Park (KGMP, Agri. Corp., Cheongdo, Korea). Samples without damage on the fruiting body and of uniform size and color were selected and packaged with a sterile biaxially oriented polypropylene (BOPP) film by 400±10 g, to be transferred within 12 h to the 60Co gamma irradiation facility.
At the 60Co gamma irradiation facility (150 TBq capacity, ACEL, MDS Nordion Inc., Ottawa, Ontario, Canada) in the Advanced Radiation Technology Institute of Korea Atomic Energy Research Institute (KAERI, Jeongeup, Korea), king oyster mushroom samples in a box were irradiated at a constant dose rate (1 kGy/h) to reach 0.5, 1, 2, and 3 kGy. To identify the actual absorbed dose, alanine dosimeters (Bruker Instruments, Rheinstetten, Baden-Württemberg, Germany) attached to the upper, middle, and lower parts of each box before irradiation were used in the analysis by an electron paramagnetic resonance analyzer (e-scan alanine analyzer, Bruker Instruments). The error range between the target and actual absorbed doses was within 5%. The samples after gamma irradiation were stored for 28 days in a constant climate chamber (TH-DG-150, Jeio tech Co., Daejeon, Korea) at 4±1°C and 80% relative humidity, for the subsequent analysis.
In the microbiological analysis, 10 g of the fruiting body of king oyster mushroom and 90 g of sterile water were placed in a stomacher bag (BagFilter 400S, Interscience, Saint-Nom-la-Bretèche, France) for 1-min homogenization using a stomacher (bag mixer 400, Interscience), after which the suspension was serially diluted at 10× for analysis. The total aerobic bacteria were measured after inoculation on a plate count agar (Difco Co., Detroit, Michigan, USA), which was cultured at 37°C for 24 h. To measure Pseudomonas spp., a cetrimide fucidin cephaloridine agar (Oxoid) with the addition of a selective supplement (SR103, Oxoid, Basingstoke, Hampshire, England) was used in inoculation, followed by 38 h culture at 25°C. To measure yeasts and molds, a potato dextrose agar (pH 3.5, Difco Co.) with the addition of 10% L-(+) tartaric acid was used in inoculation, followed by a 48 h culture at 25°C. All microbial colonies were manually counted on agar plates ranging from 15-300 CFU/g and converted to log CFU/g. The limit of detection was less than 1 log CFU/g.
To measure reducing sugars, 5 g of the stem of king oyster mushroom was mixed with 20 mL of distilled water, and the mixture was homogenized (50 rpm, 1 min) using a homogenizer (DIAX 900, Heidolph Instruments GmbH & Co. KG, Schwabach, Bavaria, Germany). After heating in a water bath (100°C, 3 h) and subsequent centrifugation (3,000 rpm, 5 min, 4°C) using a centrifuge (Allegra 25R, Beckman Coulter, Brea, California, USA), the supernatant was collected and filtered using a 0.45-μm syringe filter (Rainbow Co., Ltd., Dongguan, Guangdong, China). To 0.5 mL of the filtrate, 1.5 mL of 3,5-dinitrosalicylic acid (DNS, Sigma-Aldrich, St Louis, MO, USA) was added, and the mixture after a reaction in a water bath (100°C, 5 min) was left to stand at room temperature. The absorbance was measured at 550 nm using a microplate reader (Varioskan Flash, Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA), and glucose (Sigma-Aldrich) was used in generating the standard curve.
The color of king oyster mushrooms was using a colorimeter (CM-5, Konica Minolta, Chiyoda, Tokyo, Japan). After measuring the lightness (L), redness (a), and yellowness (b) of the stem of the mushroom, the chrominance (ΔE) was calculated using the equation:
To measure firmness, the stem ofking oyster mushroom was applied to a texture analyzer (TA-XT2i, Stable Micro Systems Ltd., Godalming, Surrey, England), which was set in the following conditions: a circular probe of 10 mm diameter (∅10 mm), pre-speed 120 mm/min, test-speed 30 mm/min, post-speed 120 mm/min, and distance 10 mm, for a compression test. A texture exponent software (Version 6.1.11.0., Stable Micro Systems Ltd.) was used in data analysis, and the highest resistance value on the force-time curve was expressed in Newton (N).
In this study, Enzyme extraction followed the method proposed by Jung et al. (2004) with modifications. The wrinkled portion on the cap of the king oyster mushroom was pulverized in 200 mg liquid nitrogen, and the sample was treated with 1 mL of 100 mM potassium phosphate buffer (pH 7.0, Sigma-Aldrich) containing 2 mM ethylene-diaminetetraacetic acid (EDTA, Sigma-Aldrich), 1% polyvinylpyrrolidone (PVP, Sigma-Aldrich) and 1 mM phenylmethylsulfonyl fluoride (PMSF, Sigma-Aldrich). An enzyme extract was obtained after a 20 min reaction at 4°C. The extract was centrifuged (10,000 rpm, 5 min, 4°C), after which the supernatant was filtered using a 0.45 μm syringe filter. The filtrate was used in subsequent analysis.
To examine tyrosinase activity, the methods of Lopez-Tejedor and Palomo (2018) were followed. 50 μL of mushroom extract was mixed with 450 μL of 0.1 M sodium phosphate buffer (pH 7.0, Sigma-Aldrich) and 500 μL of 1 mM l-3,4-dihydroxyphenylalanine (L-DOPA, Sigma-Aldrich). The mixture was left to react in a water bath (25°C, 2 h), after which measurements were performed using a microplate reader. Tyrosinase activity was expressed in the unit (U) of the melanin (Sigma-Aldrich) standard curve.
To examine β-glucanase activity, the laminarin analysis method, as described by Hong et al. (2017) was followed. 50 μL of the mushroom-extract was mixed with 425 μL of 50 mM sodium acetate buffer (pH 5.0, Junsei Chemical Co., Ltd., Chuo-ku, Tokyo, Japan) and 25 μL of laminarin (10 mg/mL, Sigma-Aldrich), and the mixture was left to react in a water bath (37°C, 2 h). After adding 1.5 mL of DNS (Sigma-Aldrich), the mixture was heated in a water bath (100°C, 5 min) and left to stand at room temperature. Then, the absorbance at 550 nm was measured using a microplate reader. β-Glucanase activity was expressed in the unit (U) of the glucose standard curve.
In microstructure analysis, following the methods of Akram et al. (2012) with modifications, the surface tissues from the stem of king oyster mushrooms at 28 d of storage were freeze-dried. The freeze-dried tissue samples were gold coated using a carbon coater (108-CA, JEOL Ltd., Akishima, Tokyo, Japan), and the microstructure was analyzed using a scanning electron microscope [(SEM) JSM-633F, JEOL Ltd.].
Experimental data were expressed as mean±SD, and a statistical program Minitab version 20.1.3 (Minitab LLC, State College, Pennsylvania, USA) was used to perform statistical analysis. Analysis of variance (ANOVA) was applied, and the post-hoc test on the significance of sample variation was Tukey’s multiple comparison test set at p<0.05.
3. Results and discussion
Table 1 presents the microbiological changes in the king oyster mushrooms based on storage period (0, 7, 14, 21, and 28 days) after gamma irradiation (0, 0.5, 1, 2, and 3 kGy). At the 0 day of storage, the counts of total aerobic bacteria, Pseudomonas spp., and yeast and molds in the non-irradiated group were 5.81±0.04, 5.23±0.10, and 4.84±0.01 log CFU/g, respectively. On the contrary, in the irradiated group, all microbial counts decreased significantly as the irradiation dose increased, and aerobic bacteria or Pseudomonas spp. was not detected for gamma irradiation ≥1 kGy and no yeasts or molds was detected for gamma irradiation ≥2 kGy. Moreover, despite an increasing trend in microbial counts with an increasing storage period, the microbial counts in the irradiated group at gamma irradiation ≥2 kGy were lower than those in the non-irradiated group at the 0 day of storage. The sterilization mechanism in food irradiation has been reported to directly damage cellular components such as DNA, carbohydrates, and lipids, or indirectly damage through reactive oxygen species and free radicals generated upon the reaction between microbial cells and water in foods (Bisht et al., 2021). The level of sterilization has been shown to rely on microbiological properties such as cytosolic water content, DNA size, and antioxidant enzymes as well as the water content in foods, water activity, and temperature (Kim, 2006). In previous studies, gamma irradiation within the range of 1-2 kGy or higher has been reported to reduce the microbial counts to a level below the limit of detection in Agaricus bisporus (Dong et al., 2022; Koorapati et al., 2004; Wani et al., 2009), Lentinula edodes (Jiang et al., 2010), and Flammulina Velutipes (Yeom et al., 2023). Hence, gamma irradiation at ≥2 kGy was the effective dose to reduce the level of microbial contamination during post-harvest storage of king oyster mushrooms.
1) Values with the same column with different superscripts (a-d) indicates significant differences (p<0.05) by Tukey’s multiple comparison test (n=3).
Table 2 presents the changes in reducing sugars of gamma irradiated king oyster mushrooms during storage. The contents of reducing sugars did not vary significantly between the non-irradiated and irradiated groups at the 0 day of storage. However the reducing sugars increased significantly in the non-irradiated group from 14 days to 28 days, and no increase was observed in the irradiated group. As observed in our findings, a previous study on the reducing sugars in Agaricus bisporus after irradiation has shown that the contents of reducing sugars decrease significantly from 16 days of storage (Duan et al., 2010). Reducing sugars in mushrooms are indicator for measuring post-harvest quality reduction as they increase primarily because of microbial growth during storage (Jiang et al., 2013; Li et al., 2021). Thus, the result demonstrates that the gamma irradiation treatment inhibits microbial growth of king oyster mushrooms, which led to a lower content of reducing sugars that was increased during storage.
Tyrosinase activity in king oyster mushrooms after gamma irradiation decreased significantly from 0 day to 21 days of storage in the irradiated group, compared with the non-irradiated group (Table 3). The treatment of 2 kGy gamma irradiation was shown to decrease the tyrosinase activity in Agaricus bisporus up to 12 days of storage, as reported in the literature (Beaulieu et al., 1999). Tyrosinase engages in the early stage of melanin biosynthesis in microorganisms, plants, and animals. Notably, it is one of the primary enzymes that reduce the commercial values of mushrooms during distribution by causing enzymatic browning of post-harvest mushrooms (Mahdavi et al., 2022; Soler-Rivas et al., 1998). Therefore, by inhibiting tyrosinase activity, gamma irradiation can delay the enzymatic browning of king oyster mushrooms during storage.
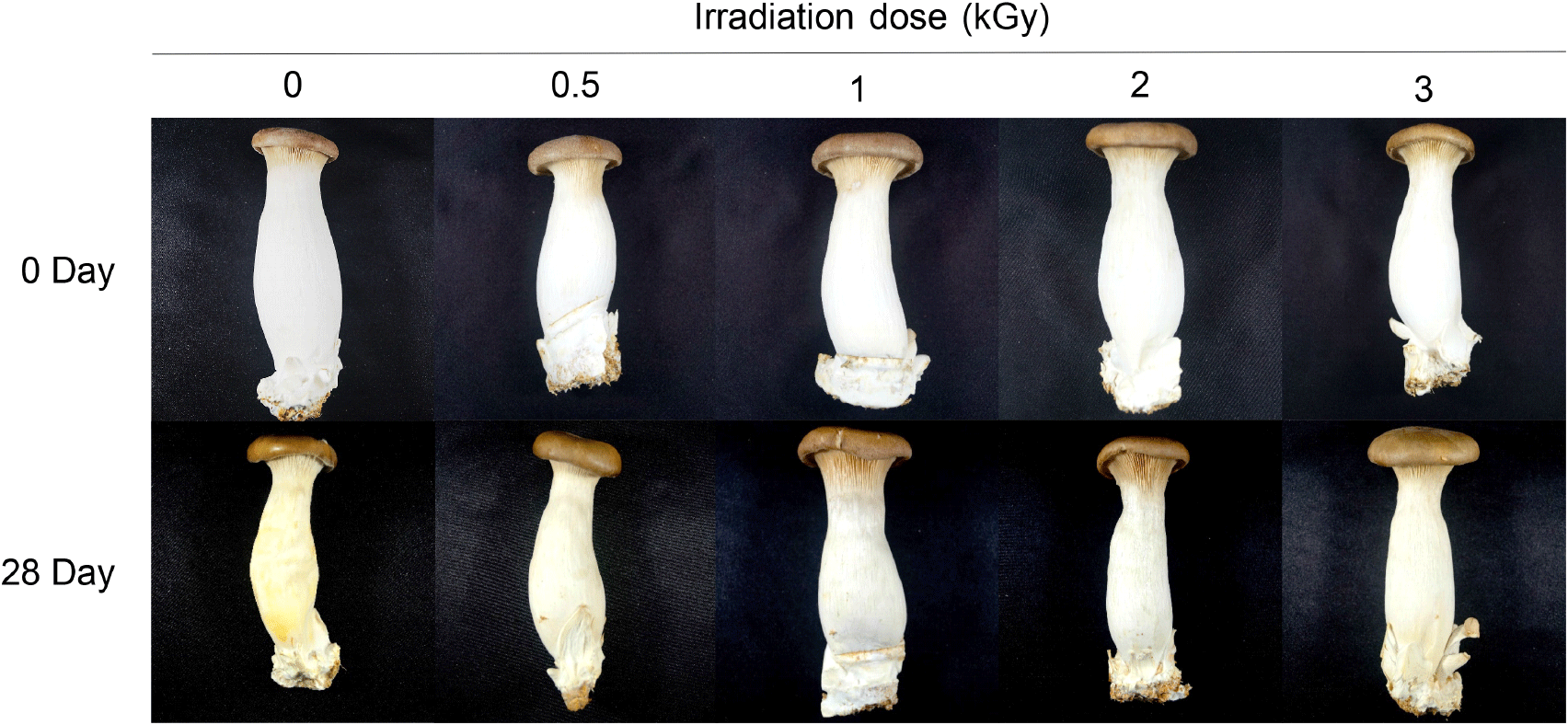
Hunter’s color values of gamma-irradiated king oyster mushrooms based on the storage period is presented in Table 4 and Fig. 1. At the 0 day of storage, the non-irradiated and irradiated groups did not vary significantly. However, with an increase in storage period, both groups exhibited a decrease in lightness and an increased in redness and yellowness, which indicated the color of king oyster mushrooms changed to brown. The ΔE started to decrease significantly in the irradiated group from 21 days of storage, and the level at 28 days of storage was 14.47±0.47 in the non-irradiated group and 5.74±0.28, 4.99±0.35, 6.54±0.72, and 6.28±0.26 in the irradiated group at 0.5, 1, 2, and 3 kGy, respectively. This implied that gamma irradiation could suppress the browning of king oyster mushrooms during storage. The treatment of Agaricus bisporus (Koorapati et al., 2004; Mami et al., 2014) and Flammulina velutipes (Yeom et al., 2023) through irradiation led to the same suppression of browning as observed in this study. Browning occurs in mushrooms during post-harvest storage due to increased levels of redness and yellowness with a decreasein lightness, and the process is accelerated through microbial growth and enzymatic activity (Fu et al., 2022). However, food irradiation has been reported to suppress the browning of mushrooms by effectively controlling microbial contamination and reducing the activities of enzymes such as tyrosinase (Kortei et al., 2015). Thus, gamma irradiation in this study effectively delayed the browning of king oyster mushrooms during post-harvest storage (28 days) by controlling microbial contamination and inhibiting tyrosinase.
Changes in β-glucanase activity in king oyster mushrooms after gamma irradiation during storage are presented in Table 5. Irrespective of gamma irradiation, β-glucanase activity did not change significantly in all the samples and a decreasing trend was found during storage. β-Glucanase is known to degrade β-glucan, a primary component of cell walls, into monomers, thereby causing structural changes (Kaur et al., 2020). Few studies on changes in β-glucanase activity in crops have shown that no significant difference is observed in pears after UV-C irradiation (Li et al., 2010), whereas the same treatment can increase or decrease the β-glucanase activity in strawberries (Araque et al., 2019; Pombo et al., 2011). In mangoes, the β-glucanase activity decreased immediately after electron beam irradiation but increased from 12 days of storage in the irradiated mangoes (Uthairatanakij et al., 2021). Therefore, β-glucanase activity may vary across different crops and based on the source of irradiation. However, for king oyster mushrooms, gamma irradiation did not affect β-glucanase activity during storage.
Table 6 presents the changes in the firmness of the gamma-irradiated king oyster mushrooms during storage. Immediately after gamma irradiation at ≥2 kGy, a significant reduction in firmness was observed. Firmness was also shown to significantly decrease during storage in both non-irradiated and irradiated groups. The rate of reduction indicated a steep decline in the non-irradiated samples but a gradual decrease in the irradiated samples. Specifically, the rate of reduction in firmness at 28 days of storage was 80.59±1.89% in the non-irradiated samples and 42.80±1.28, 34.57±1.13, 31.05±3.24, and 39.73±0.94% in the irradiated samples at 0.5, 1, 2, and 3 kGy, respectively, suggesting that softening of mushrooms was prevented by gamma irradiation. Firmness increased significantly by gamma irradiation at 1 and 1.5 kGy from 8 days to 20 days of storage in the case of Lentinula edodes and by electron beam irradiation at ≤4 kGy to 16 days of storage in the case of Agaricus bisporus. While firmness is an important quality property that confers the unique texture of mushrooms, it has been reported to be significantly influenced by microbial growth or digestive enzymes produced in mushrooms (Jiang et al., 2010). Therefore, our study result shows that gamma irradiation can inhibit microbial growth, thereby delaying the softening or firmness reduction of king oyster mushrooms during post-harvest storage.
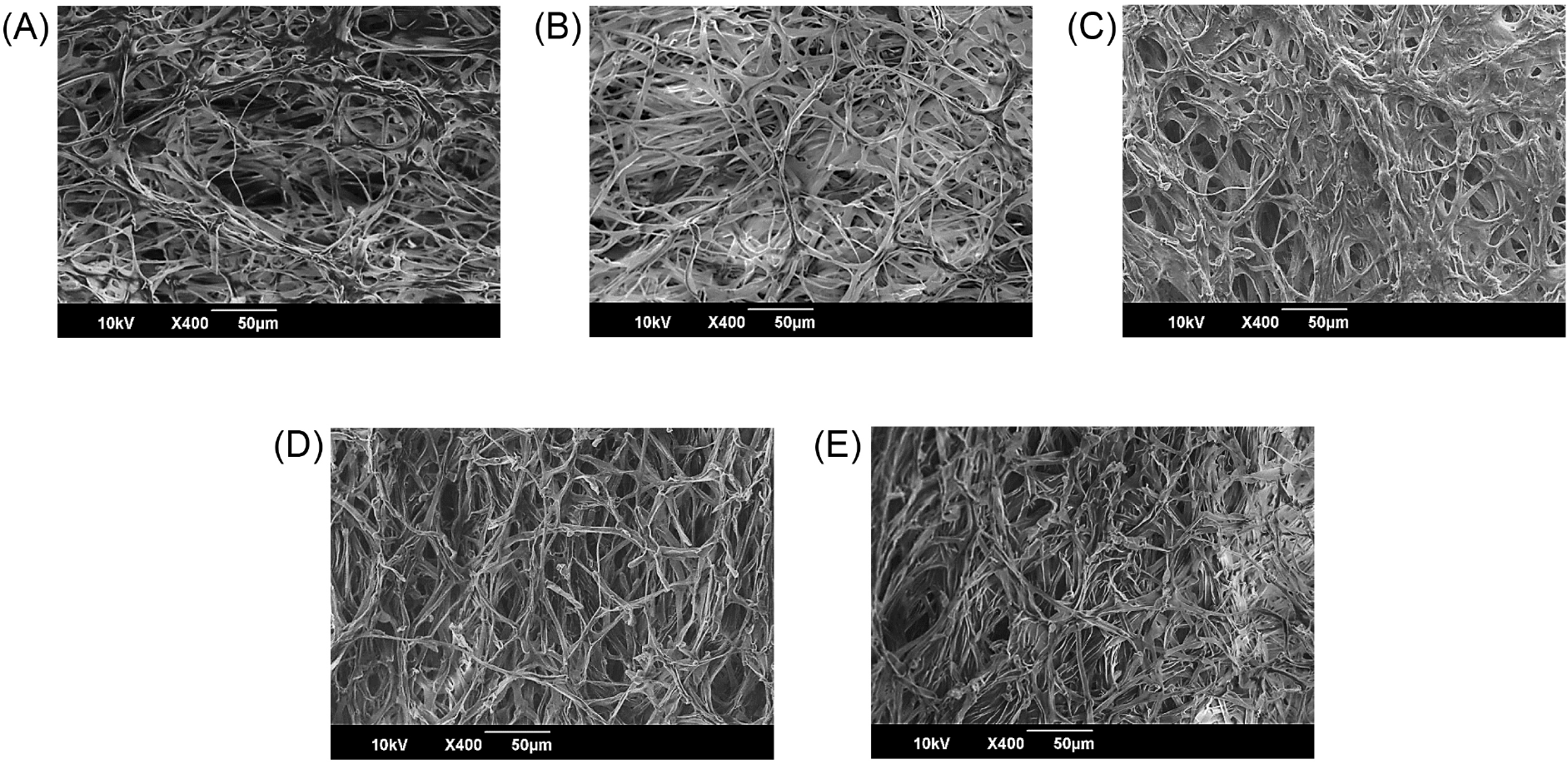
To examine the physical structural changes in gamma-irradiated king oyster mushrooms after 28 d storage, a microstructure analysis was performed using an SEM. The result confirmed that, compared to those in the non-irradiated samples, the intercellular pores in the irradiated samples were relatively small (Fig. 2). Generally, changes in the microstructure of the mushroom result from extended pores across cells due to microbial growth and enzymatic degradation of cell wall components. Notably, these changes are closely related to those in mushroom firmness (Zivanovic et al., 2000). Our study findings show that gamma irradiation can prevent microstructural changes in king oyster mushrooms to certain degrees.
4. Conclusions
The effects of gamma irradiation (0, 0.5, 1, 2, and 3 kGy) on the microbiological and physicochemical properties of king oyster mushrooms were investigated and the optimal irradiation dose was determined. In the microbiological analysis, the total aerobic bacteria, Pseudomonas spp., and yeasts and molds significantly decreased as the irradiation dose increased. In particular, gamma irradiation at ≥2 kGy reduced the microbial count effectively up to the level of non-irradiated samples at the 0 day of storage. A physicochemical quality evaluation showed that gamma irradiation significantly suppressed the browning during post-harvest storage. This could be attributed to the reduction in tyrosinase activity. The result on firmness indicated that gamma irradiation could delay the softening of king oyster mushrooms for during 28 days of storage. In the microstructure analysis, more reduced pores were observed in the irradiated group than in the non-irradiated group. In conclusion, gamma irradiation at ≥2 kGy was the most effective irradiation dose that could control microbial contamination of king oyster mushrooms during storage for 28 days after harvest while delaying the browning and softening phenomena.